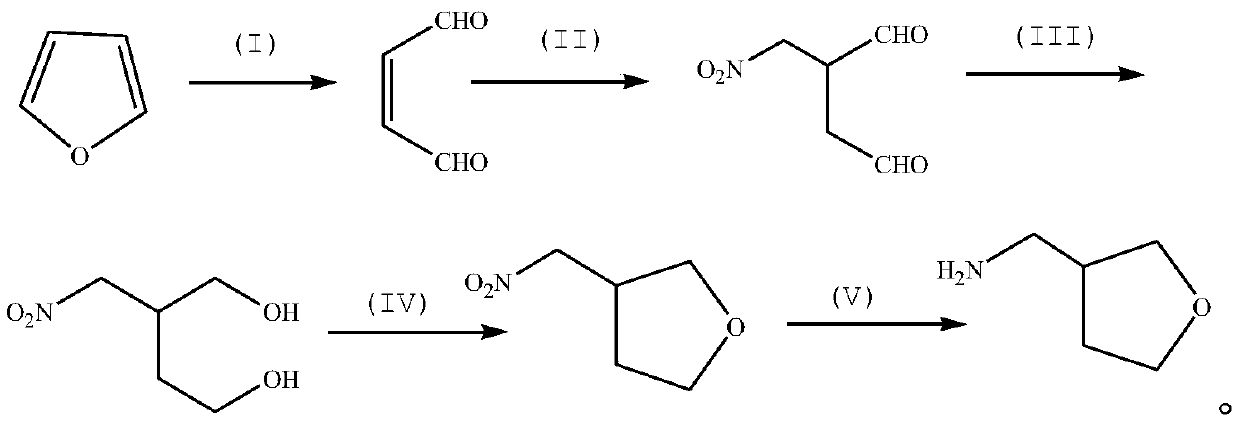
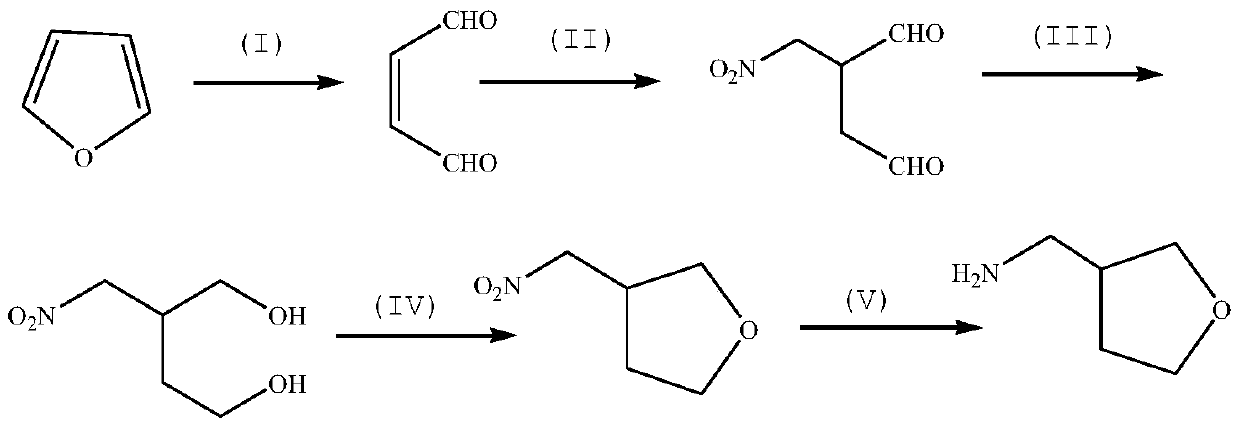
Method for synthesizing 3-aminomethyl tetrahydrofuran by taking furan as raw material
A technology of aminomethyltetrahydrofuran and nitromethyl, which is applied in the field of synthesizing 3-aminomethyltetrahydrofuran, can solve the problems of unsuitability for industrial production, long synthesis route and high total yield, and achieves low raw material price and low production cost , the effect of short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of TS-1 catalyst for synthesis of 1,4-butenedialdehyde by oxidative ring opening of furan.
[0035] According to US Patent 4410501, 455 grams of tetraethyl silicate are placed in a single-necked flask equipped with a stirrer and kept in a carbon dioxide-free state, 15 grams of titanium (IV) tetraethoxide are added, and then 800 grams of 25% by weight are gradually added Tetrapropylammonium hydroxide solution (without inorganic base). The mixture was stirred for about 1 hour, and then heating was started carefully to accelerate the hydrolysis and evaporate the liberated ethanol. After about 5 hours at 80-90°C, the ethanol has completely evaporated to dryness. The volume was increased to 1.5 liters with distilled water, and the milky white homogeneous solution was transferred to an autoclave equipped with a stirrer. The mixture was heated to 175°C and kept stirring at this temperature under its own pressure for 10 days. Then the autoclave is cooled, the reacti...
Embodiment 2
[0037] Synthesis of 1,4-butenedialdehyde.
[0038] 300 ml of acetonitrile, 21.0 g (0.30 mol) of furan, TS-1 (3 g) and hydrogen peroxide (35%, 0.36 mmol) were added to a 1000 ml single-necked flask. The mixture was stirred at room temperature for 2 hours, at which time the peroxide disappeared (detected with saturated potassium iodide aqueous solution). 200 ml of water was added to the reaction, the aqueous layer was extracted three times with dichloromethane, the organic layers were combined, dried over magnesium sulfate, filtered, and concentrated to obtain the product 1,4-butenedialdehyde, which was directly used in the next step 2-nitromethyl -1,4-Butanedialdehyde synthesis reaction. 1 H NMR(400MHz, CDCl 3 )δ9.70 (m, 2H), 7.70-7.66 (m, 2H).
Embodiment 3
[0040] Synthesis of 2-nitromethyl-1,4-butanedialdehyde.
[0041] Add proline (1.15g, 0.01mol) to nitromethane (54g, 0.9mol), then add the 1,4-butenedialdehyde obtained above, stir at room temperature for 12h, and then add 100ml of ice water , Extracted with ether three times, 100 ml each time, combined the organic layers, dried and concentrated to obtain the product 2-nitromethyl-1,4-butanedialdehyde directly used in the next step 2-nitromethyl-1,4- Synthesis of butanediol.
[0042] NMR (400MHz, CDCl 3 )δ9.70(s,2H),4.70-4.66(d,J=8.0Hz,2H),2.71-2.65(m,1H), 2.50-2.41(m,2H),2.37-2.30(m,1H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com