Preparation method of polyacrylic acid-tocopherol succinate self-assembly drug loading system for slow drug release
A technology of tocopheryl succinate and polyacrylic acid, which is applied in the field of material synthesis and biomedicine, can solve the problems of unsatisfactory efficacy and achieve the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of polyacrylic acid-tocopheryl succinate self-assembled drug loading system includes the following steps:
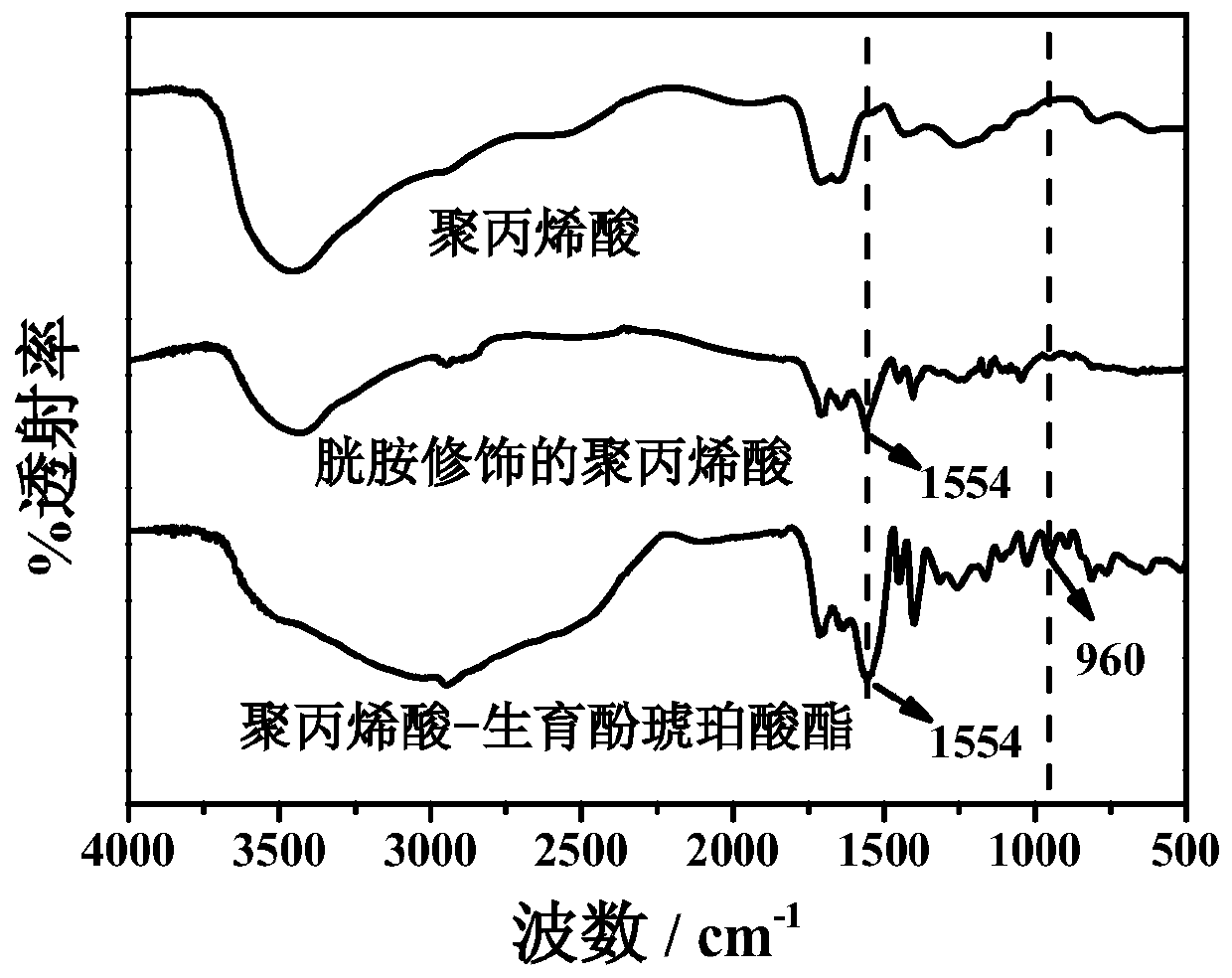
[0024] (1) Mix 0.2g polyacrylic acid and 50mL pH value of 5.6 phosphate buffer solution, then add 0.247g N-hydroxysuccinimide and 0.411g 1-ethyl-(3-dimethylaminopropyl) carbon Diimine hydrochloride, stirred at room temperature 25°C for 1 hour; then added 1.4 g of cystamine hydrochloride, and continued to stir for 24 hours; dialyzed for three days to remove unreacted substances, then centrifuged to remove the precipitate, and freeze-dried the supernatant The cystamine-modified polyacrylic acid can be obtained immediately.
[0025] (2) Dissolve 30mg of tocopheryl succinate in 10mL of N,N-dimethylformamide, then add 6.5mg of N-hydroxysuccinimide and 10.9mg of 1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride, stirred at room temperature 25°C for 1 hour; then a certain amount of cystamine-modified polyacrylic acid prepared in step (1) was di...
Embodiment 2
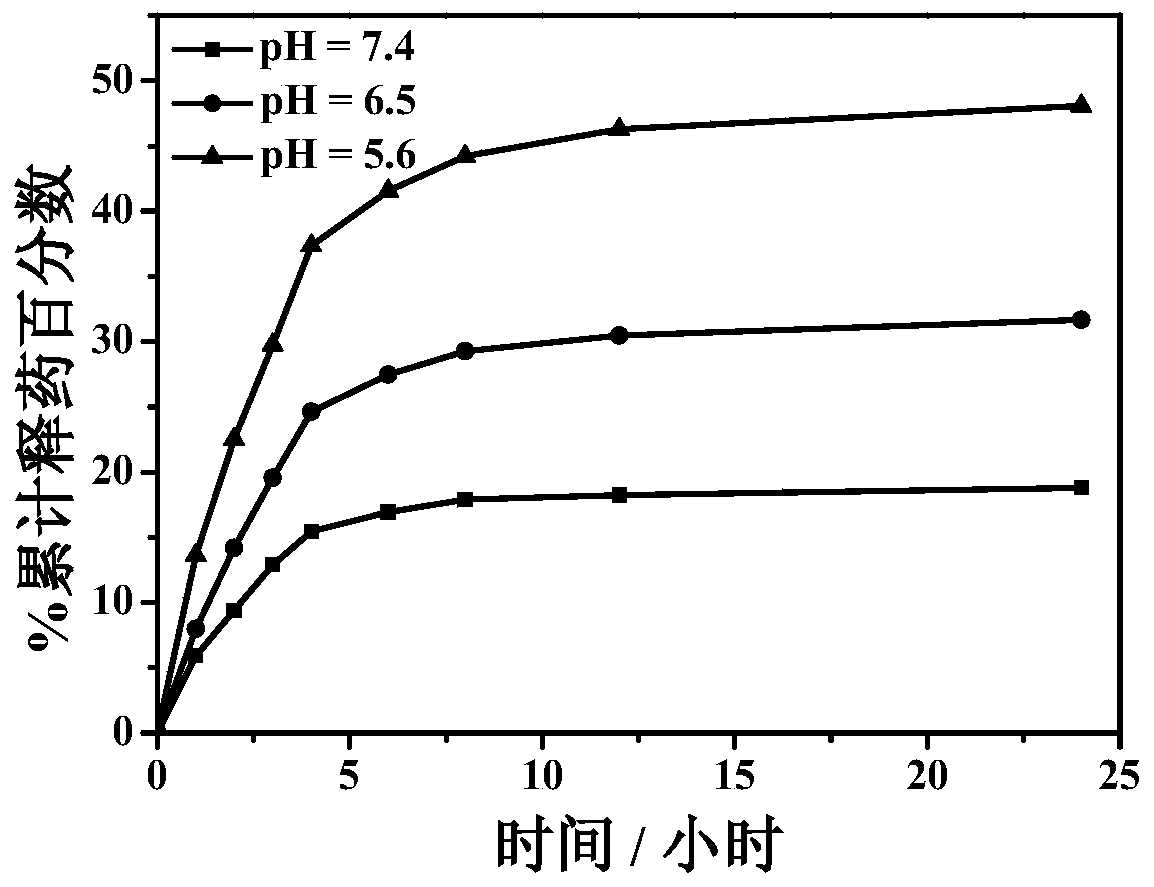
[0030] Drug release in vitro at different pH values involves the following steps:
[0031] The preparation process of the polyacrylic acid-tocopheryl succinate self-assembled drug-loading system is the same as that of Example 1.
[0032] (1) Weigh 20 mg of the drug-loaded composite material, place them in dialysis bags, and place the dialysis bags in 50 mL of phosphate buffer solution with different pH; magnetically stir at a temperature of 37°C to release the drug in vitro; The pH of the phosphate buffered saline was 5.6, 6.5 and 7.4 and the release was 24 hours.
[0033] (2) Take a sample every 1 hour for the first 4 hours, take a sample every 2 hours for 4 to 12 hours, and take a sample every 12 hours for 12 to 24 hours. Take 3 mL of solution for each sample, and measure the released formazan The amount of methotrexate was supplemented with 3 mL of fresh phosphate buffered saline solution; the concentration of methotrexate was measured at 302nm using an ultraviolet spect...
Embodiment 3
[0036] In vitro drug release under glutathione-stimulated conditions involves the following steps:
[0037] The preparation process of the polyacrylic acid-tocopheryl succinate self-assembled drug-loading system is the same as that of Example 1.
[0038] (1) Weigh 20 mg of the drug-loaded composite material and place it in a dialysis bag, put the dialysis bag in 50 mL of phosphate buffer solution with a pH of 5.6 and containing 10 mM glutathione, and perform magnetic stirring. Released for 24 hours.
[0039] (2) Take a sample every 1 hour for the first 4 hours, take a sample every 2 hours for 4 to 12 hours, and take a sample every 12 hours for 12 to 24 hours. Take 3 mL of solution for each sample, and measure the released formazan The amount of methotrexate was supplemented with 3 mL of fresh phosphate buffered saline solution; the concentration of methotrexate was measured at 302nm using an ultraviolet spectrophotometer, and the cumulative drug release percentage at differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com