A kind of preparation method of trans tetrasubstituted olefin derivative
A tetra-substituted and derivative technology, applied in the field of preparation of trans-tetra-substituted alkene derivatives, can solve problems such as theoretical difficulties, and achieve the effects of wide substrate range, good regioselectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
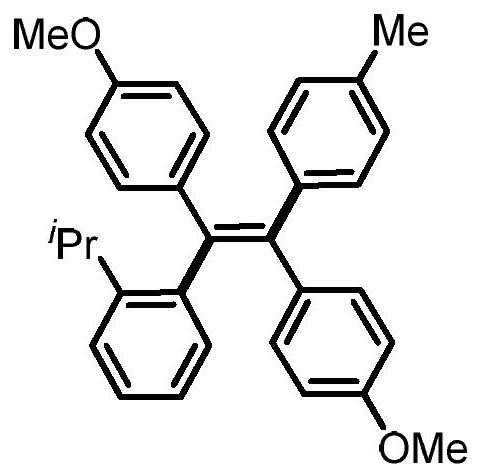
[0024] Preparation of (Z)-4,4'-(1-(o-tolyl)-2-(p-tolyl)ethylene-1,2-diyl)bis(methoxybenzene)
[0025]
[0026] 0.3mmol of sodium carbonate, 0.1mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of tetrakistriphenylphosphine palladium, 0.005mmol of bis(2-diphenylphosphophenyl) ether, 4- Add 0.2mmol of methylphenylboronic acid, 0.3mmol of 2-isopropyl iodobenzene and 1mL of N,N-dimethylformamide into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, and place it in an oil bath at 120°C. Reacted for 24h; cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times with water, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 34.9 mg of the target product with a yield of 78%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.17-7.12 (m, 2H), 7.03-6.93 (...
Embodiment 2
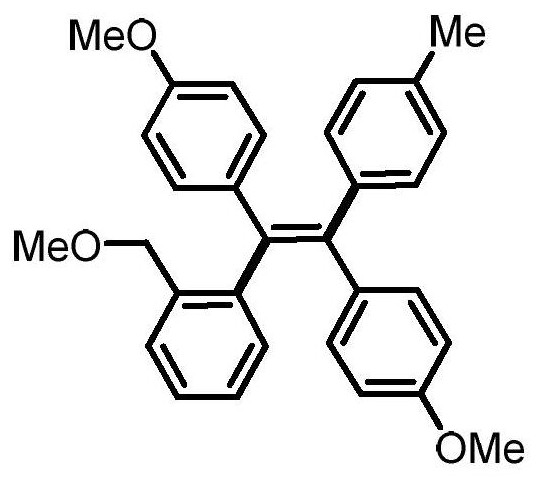
[0028] (Z)-4,4'-(1-(2-(methoxymethyl)phenyl)-2-(p-tolyl)ethylene-1,2-diyl)bis(methoxybenzene) preparation
[0029]
[0030] 0.3mmol of sodium carbonate, 0.1mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of tetrakistriphenylphosphine palladium, 0.005mmol of bis(2-diphenylphosphophenyl) ether, 4- Add 0.2mmol of methylphenylboronic acid, 0.3mmol of 1-iodo-2-(methoxymethyl)benzene and 1mL of N,N-dimethylformamide into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, and place In an oil bath at 120°C, react for 24 hours; cool to room temperature, dilute the reaction solution with ethyl acetate, wash with water three times, and wash the organic phase with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 22 mg of the target product, with a yield of 49%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.30 (dd, J = 7.8, 1.3Hz...
Embodiment 3
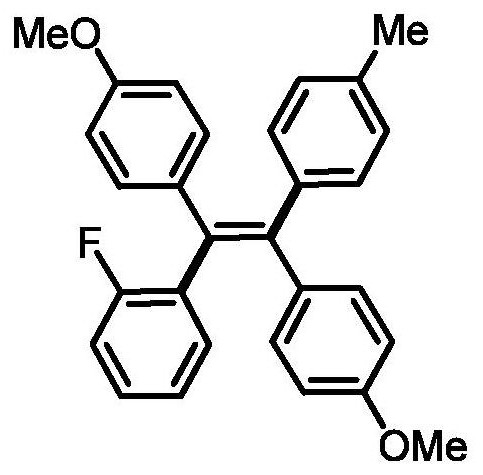
[0032] Preparation of (Z)-4,4'-(1-(2-fluorophenyl)-2-(p-tolyl)ethylene-1,2-diyl)bis(methoxybenzene)
[0033]
[0034] 0.3mmol of sodium carbonate, 0.1mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of tetrakistriphenylphosphine palladium, 0.005mmol of bis(2-diphenylphosphophenyl) ether, 4- Add 0.2mmol of methylphenylboronic acid, 0.3mmol of 2-fluoroiodobenzene and 1mL of N,N-dimethylformamide into a 15mL reaction tube, fill it with nitrogen gas repeatedly 10 times, place it in an oil bath at 120°C, and react for 24h ; Cooled to room temperature, the reaction solution was diluted with ethyl acetate, washed three times, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, concentration, and purification by thin-layer chromatography gave 19.9 mg of the target product, with a yield of 47%. The NMR and high-resolution mass spectrometry of this compound are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.11-7.03(m, 3H), 6.97-6.90(m, 10H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com