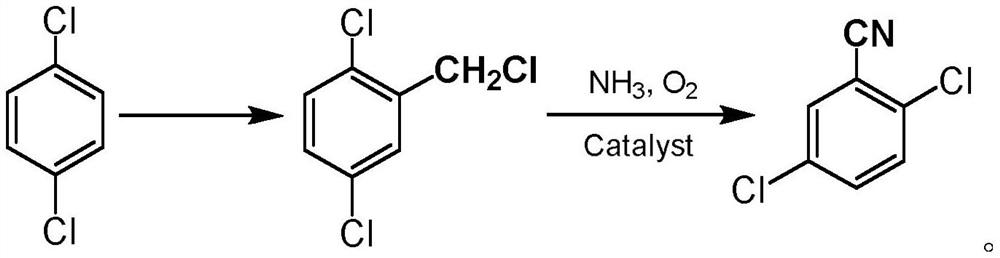
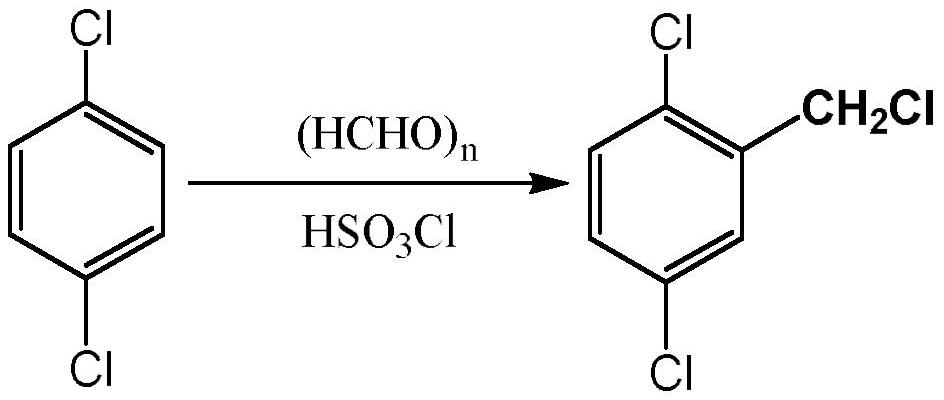
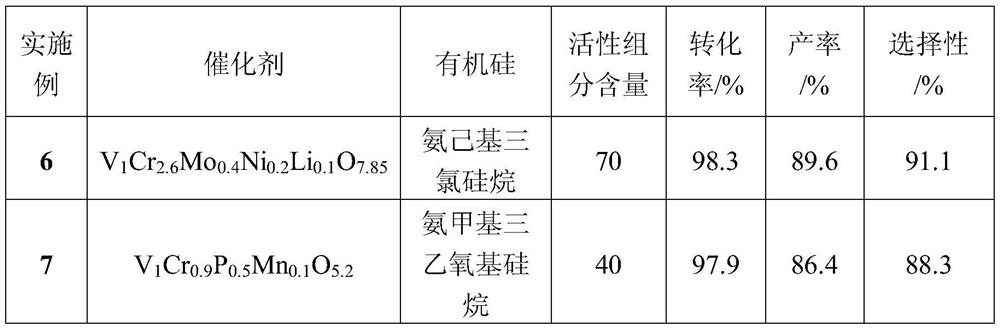
Method for preparing 2,5-dichlorobenzonitrile, special catalyst and preparation method thereof
A technology of dichlorobenzonitrile and catalyst, which is applied in the field of preparing 2,5-dichlorobenzonitrile, can solve the problems of harsh reaction conditions, long reaction time, complicated post-treatment, etc., and achieves improved selectivity, simple preparation method and environmental protection. friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Add 29.4g of p-dichlorobenzene, 7.8g of paraformaldehyde and 1.5g of formaldehyde solution in a 100ml three-necked flask, install a mechanical stirrer, connect an air condenser with a balloon on the upper end and a constant pressure tube with 14ml of chlorosulfonic acid respectively. Dropping funnel, react in ice-water bath at 0°C, slowly add chlorosulfonic acid dropwise, start the stirrer to stir, the mixture turns purple, after 1.5h dropwise addition, raise the temperature to 50°C for 48h reaction. After the reaction is over, add saturated sodium carbonate solution to completely neutralize the unreacted acid solution, then add a certain amount of methylene chloride to the flask to extract the product, and wash the mixed solution two to three times with saturated sodium chloride solution after liquid separation. The obtained organic layer solution was dried with anhydrous sodium sulfate to remove water, spin-dried the organic solvent, and distilled under red...
Embodiment 2
[0025] Add 24.5g of p-dichlorobenzene and 10g of formaldehyde solution to a 100ml three-necked flask, slowly add 30g of benzenesulfonyl chloride dropwise at 0°C, and raise the temperature to 60°C for 72h. After the reaction is over, add saturated sodium carbonate solution to completely neutralize the unreacted acid solution, then add a certain amount of methylene chloride to the flask to extract the product, and wash the mixed solution two to three times with saturated sodium chloride solution after liquid separation. The obtained organic layer solution was dried with anhydrous sodium sulfate to remove water, spin-dried the organic solvent, and distilled under reduced pressure with an oil pump. At 170° C., an oily colorless transparent liquid with a pungent smell was collected, with a yield of 78%.
Embodiment 3
[0027] Add 14.7g of p-dichlorobenzene and 9g of formaldehyde solution into a 100ml three-necked flask, slowly add 10g of p-toluenesulfonyl chloride dropwise at room temperature, and raise the temperature to 30°C for 60h. After the reaction is over, add saturated sodium carbonate solution to completely neutralize the unreacted acid solution, then add a certain amount of methylene chloride to the flask to extract the product, and wash the mixed solution two to three times with saturated sodium chloride solution after liquid separation. The obtained organic layer solution was dried with anhydrous sodium sulfate to remove water, spin-dried the organic solvent and distilled under reduced pressure with an oil pump. At 170° C., an oily colorless transparent liquid with a pungent smell was collected with a yield of 83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com