2,2-di-cyano methylene thiazole and application thereof
A biscyanomethylenethiazole and application technology are applied in the field of heterocyclic compounds and 2,2-biscyanomethylenethiazole derivatives, which can solve the problems of low photothermal conversion efficiency and achieve good photothermal conversion. Excellent efficiency, photostability and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 2,2-biscyanomethylenethiazole:
[0020] 2-(4-(4-cyanophenyl)thiazole-2(5H)-methylene)malononitrile (1.25g, 5.00mmol) and 4-(diphenylamino)benzaldehyde (1.37g, 5.00 mmol) and ammonium acetate in acetic acid were magnetically stirred at 120° C. for 48 hours under nitrogen protection. The solvent was then added dropwise to saturated sodium bicarbonate solution. After filtration, the filter residue was purified by column chromatography using a mixture of dichloromethane / methanol (18:1) as dark crude solid 0.75 g (34% yield).
[0021] Compound Characterization:
[0022] 1 H NMR(400MHz,MeOD),δ(ppm):7.58~7.59(d,4H),7.33~7.44(m,8H),7.19~7.23(t,2H),7.03~7.06(d,4H),6.68 ~6.70(d,1H).
[0023] 13 C NMR(100MHz,MeOD),δ(ppm):174.58,170.13,153.14,147.08,140.17,135.45,132.37,130.82,130.56,130.05,129.42,126.86,126.08,124.07,120.49,119.20,116.51,112.52,66.82 .
[0024] IR(v -1 ,KBr):3452,2924,2859,2199,1648,1589,1493,1320,1182,1079,838,756,703.
[0025] The above ...
Embodiment 2
[0026] Embodiment 2 (the ultraviolet absorption of 2,2-dicyano methylene thiazoles)
[0027] Prepare 2,2-biscyanomethylenethiazole DMSO solution with a concentration of 1 mM, take 10 μL of 2,2-biscyanomethylenethiazole DMSO solution, add it to a 10 mL centrifuge tube, and dilute to 10 mL with distilled water to obtain a concentration of 10 μM 2,2-Dicyanomethylenethiazole aqueous solution (containing 1% DMSO).
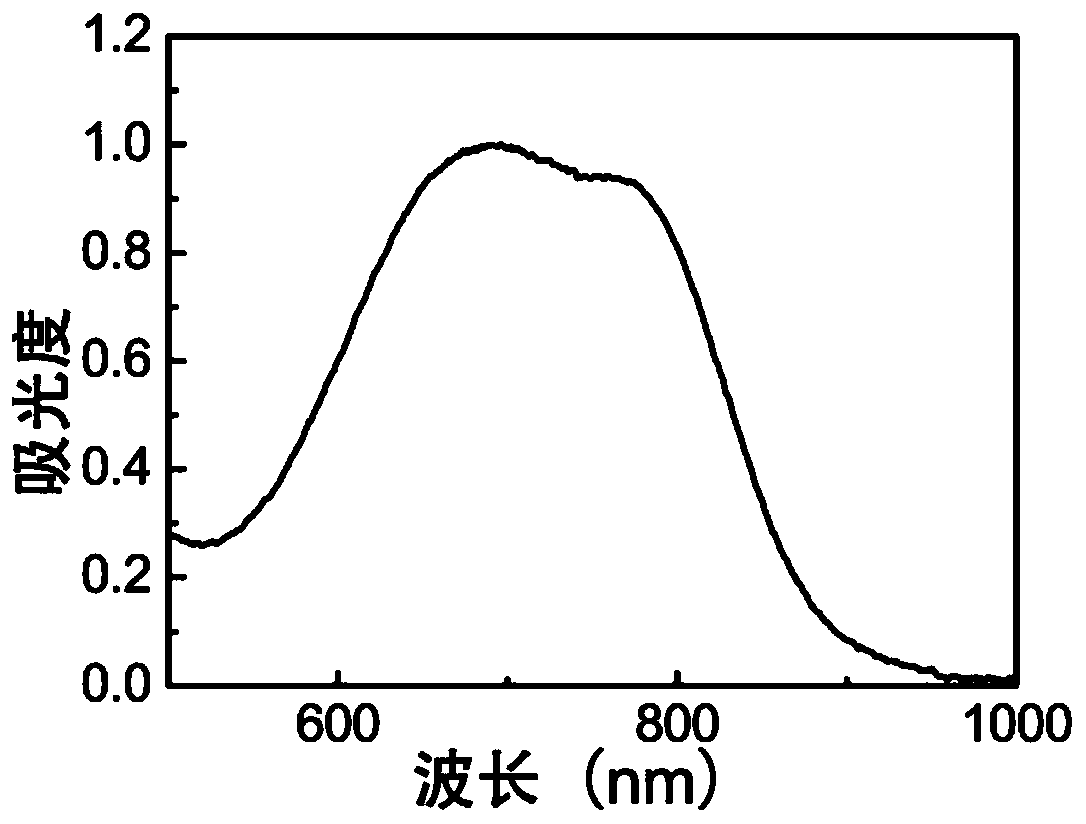
[0028] Adopt Thermofisher Evolution 300 ultraviolet absorption spectrometer to measure the ultraviolet absorption spectrum of above-mentioned 2,2-dicyano methylene thiazole aqueous solution, its result is as follows figure 1 shown. The ultraviolet absorption spectrum of 2,2-biscyanomethylenethiazole shows a strong absorption peak at 758nm, which belongs to near-infrared absorption.
Embodiment 3
[0029] Embodiment 3 (the photostability of 2,2-biscyano methylene thiazoles)
[0030] Prepare 2,2-biscyanomethylenethiazole DMSO solution with a concentration of 1 mM, take 10 μL of 2,2-biscyanomethylenethiazole DMSO solution, add it to a 10 mL centrifuge tube, and dilute to 10 mL with distilled water to obtain a concentration of 10 μM 2,2-Dicyanomethylenethiazole aqueous solution (containing 1% DMSO).
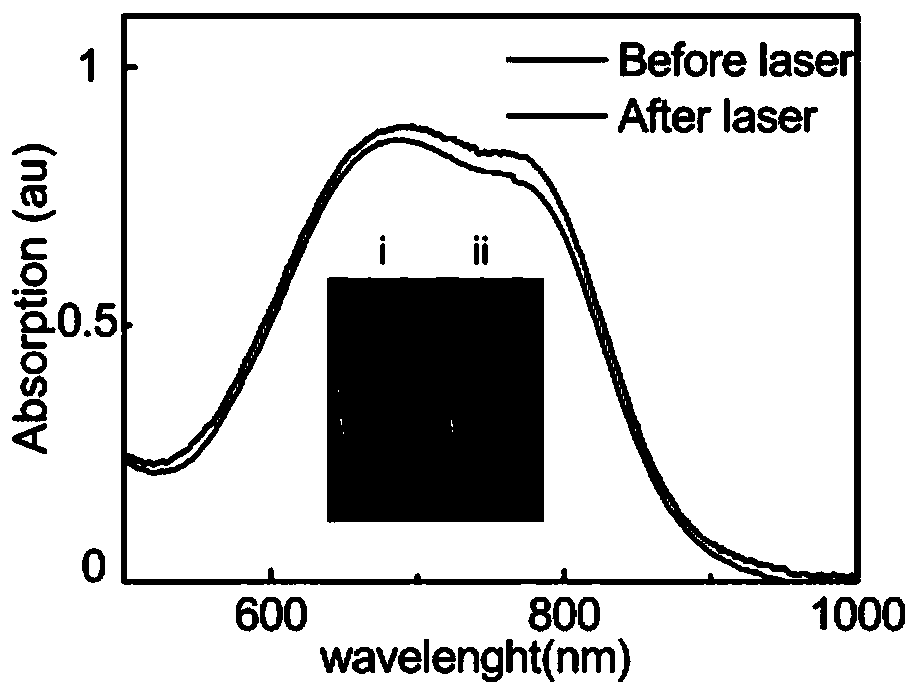
[0031] Adopt 808nm laser irradiation 2,2-dicyano methylene thiazole aqueous solution 10 minutes, adopt ThermofisherEvolution 300 ultraviolet absorption spectrometer to measure the ultraviolet absorption spectrum of above-mentioned 2,2-dicyano methylene thiazole aqueous solution, its result is as follows figure 2 shown. from figure 2 It can be known that there is little change in the ultraviolet absorption spectrum of 2,2-biscyanomethylenethiazole before and after 808nm laser irradiation, indicating that 2,2-biscyanomethylenethiazole has good photostability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com