Preparation method of efficient and stable bifunctional catalyst and application of efficient and stable bifunctional catalyst
A bifunctional catalyst and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems that bifunctional catalyst research is rarely reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
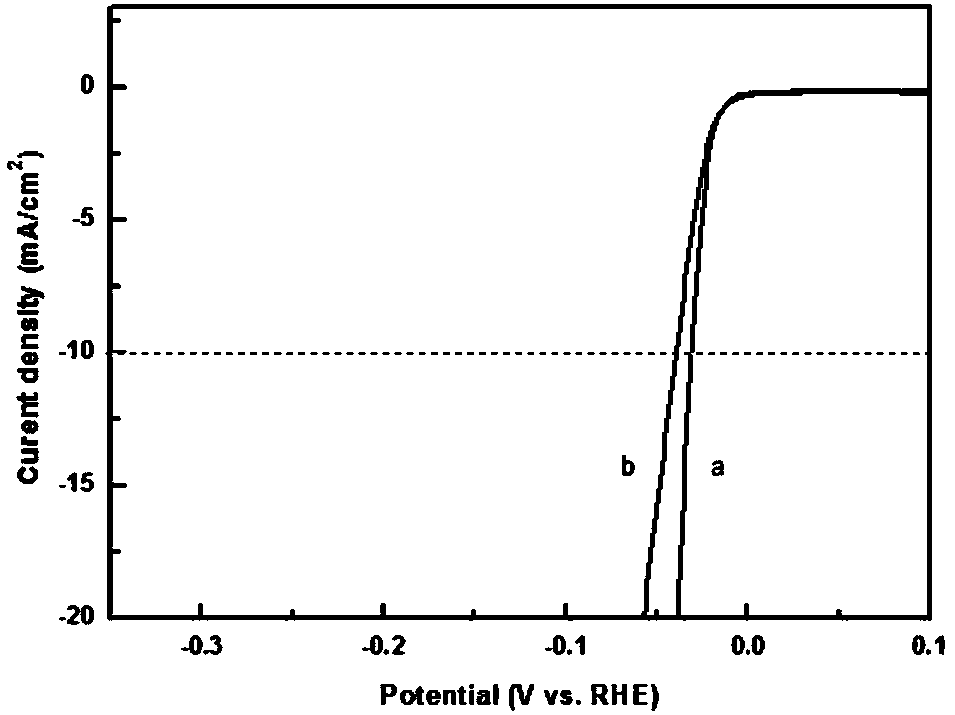
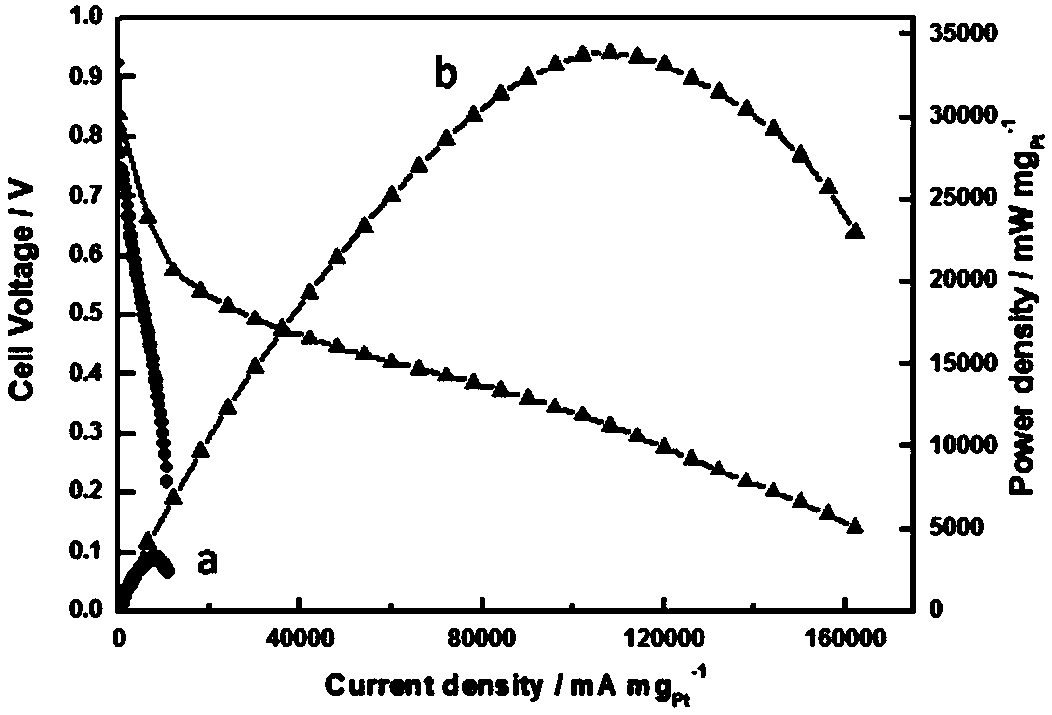
Image
Examples
Embodiment 1
[0022] (1) Weigh 30g of manganese nitrate and 10g of nickel nitrate, then stir and mix with 40g of polyethylene glycol, stir at 60°C for 48h; after stirring, add dropwise dilute nitric acid with a mass ratio of 10% to adjust the pH of the solution to 2;
[0023] (2) Put the mixed solution into a hydrothermal reaction kettle, and conduct a hydrothermal reaction at 200°C for 48 hours;
[0024] (3) After the hydrothermal reaction is completed, suction filter and wash the reactant with ultrapure water and ethanol to neutrality, and fully dry at 60°C;
[0025] (4) calcining the dry matter at high temperature for 2 hours to obtain a double metal oxide product;
[0026] (5) The product is loaded into a high-temperature and high-pressure reactor, 150° C., 3 MPa hydrogen pressure, and heated for 2 hours to obtain H 0.3 mn 0.66 Ni 0.34 o 3 .
[0027] (6) 10g H 0.3 mn 0.66 Ni 0.34 o 3 Disperse into 50mL of ethylene glycol / water solution, add dropwise 1.1mL of chloroplatinic acid...
Embodiment 2
[0029] (1) Weigh 10g of manganese nitrate, 6.6g of nickel nitrate and 12.7g of chromium nitrate, then stir and mix with polyethylene glycol, and stir at 60°C for 48h; after stirring, add dropwise dilute nitric acid with a mass ratio of 10% to adjust the pH of the solution to 2;
[0030] (2) Put the mixed solution into a hydrothermal reaction kettle, and conduct a hydrothermal reaction at 200°C for 48 hours;
[0031] (3) After the hydrothermal reaction is completed, suction filter and wash the reactant with ultrapure water and ethanol to neutrality, and fully dry at 60°C;
[0032] (4) calcining the dry matter at high temperature for 2 hours to obtain a trimetallic oxide product;
[0033] (5) The product is loaded into a high-temperature and high-pressure reactor, 150° C., 3 MPa hydrogen pressure, and heated for 4 hours to obtain H 0.5 mn 0.2 Ni 0.2 Cr 0.6 o 3 .
[0034] (6) 10g H 0.5 mn 0.2 Ni 0.2 Cr 0.6 o 3 Disperse into 50mL of ethylene glycol / water solution, add 0...
Embodiment 3
[0036] 1. Pt / H 0.3 mn 0.66 Ni 0.34 o 3 The preparation method of catalyst solution
[0037] Weigh 2.0mg Pt / H 0.3 mn 0.66 Ni 0.34 o 3 The catalyst (hereinafter referred to as the catalyst) was dispersed in 1 mL of absolute ethanol, and 11.2 μL of 5.0 wt % Nafion solution was added, and the catalyst was sonicated for 1 h to disperse the catalyst evenly.
[0038] 2. Electrode spraying method
[0039] Fix the treated glassy carbon electrode, and evenly drop the ultrasonic catalyst solution onto the glassy carbon electrode, so that it is evenly distributed in the effective area of the glassy carbon electrode, and dry the prepared glassy carbon electrode naturally to Awaiting follow-up testing.
[0040] 3. Activation of the catalyst
[0041] Using a three-electrode system, the working electrode is a prepared carbon paper electrode; the counter electrode is a carbon rod; the reference electrode is a saturated calomel electrode; the electrolyte is 0.5mol / L H 2 SO 4 solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com