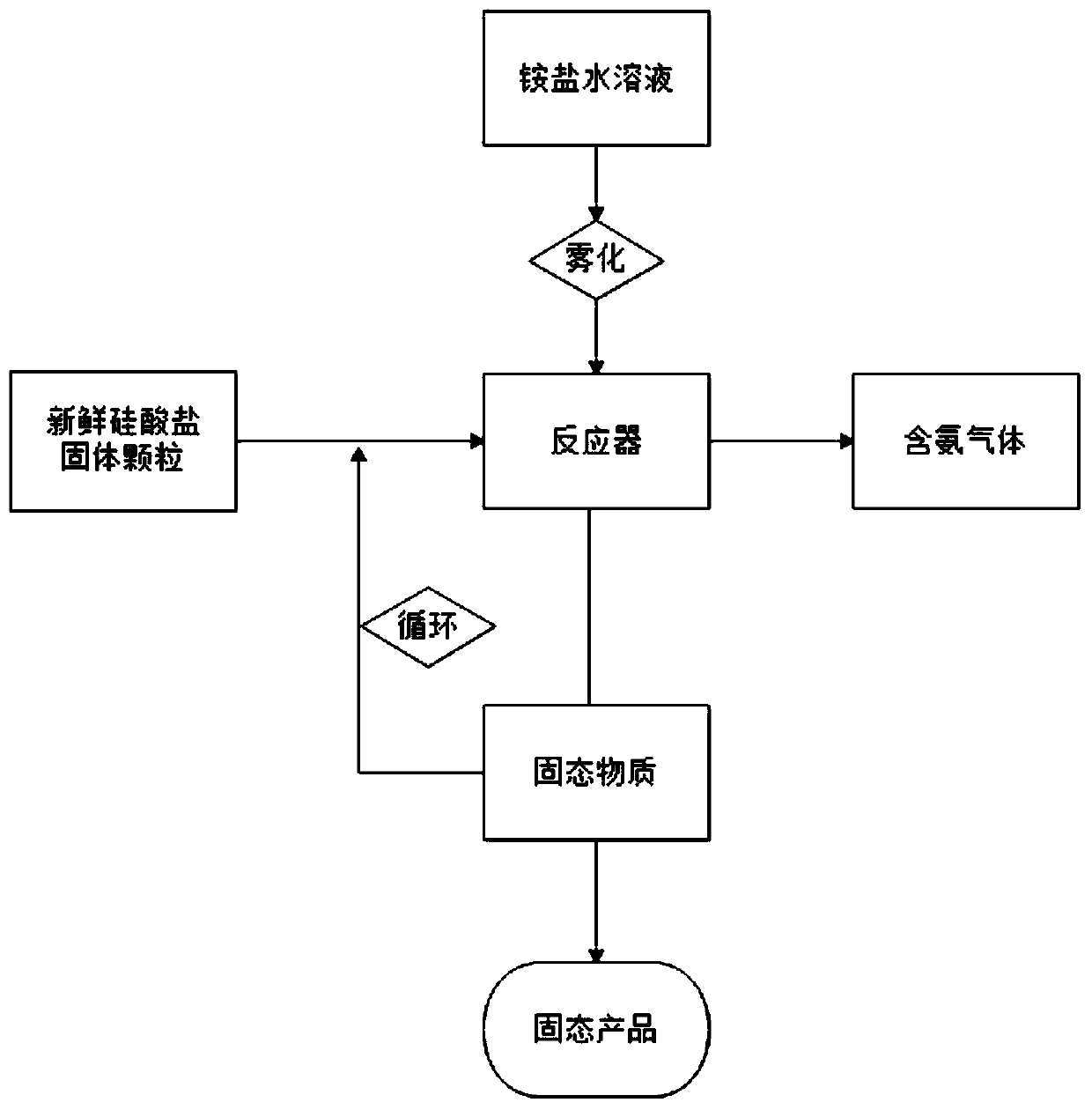
Method used for preparing ammonia gas from ammonium salt and silicate
A silicate and ammonium salt technology, applied in chemical instruments and methods, ammonia preparation/separation, ammonia compounds, etc., can solve problems such as complex processes, simplify reaction steps, avoid corrosion problems, and facilitate industrial scale-up Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
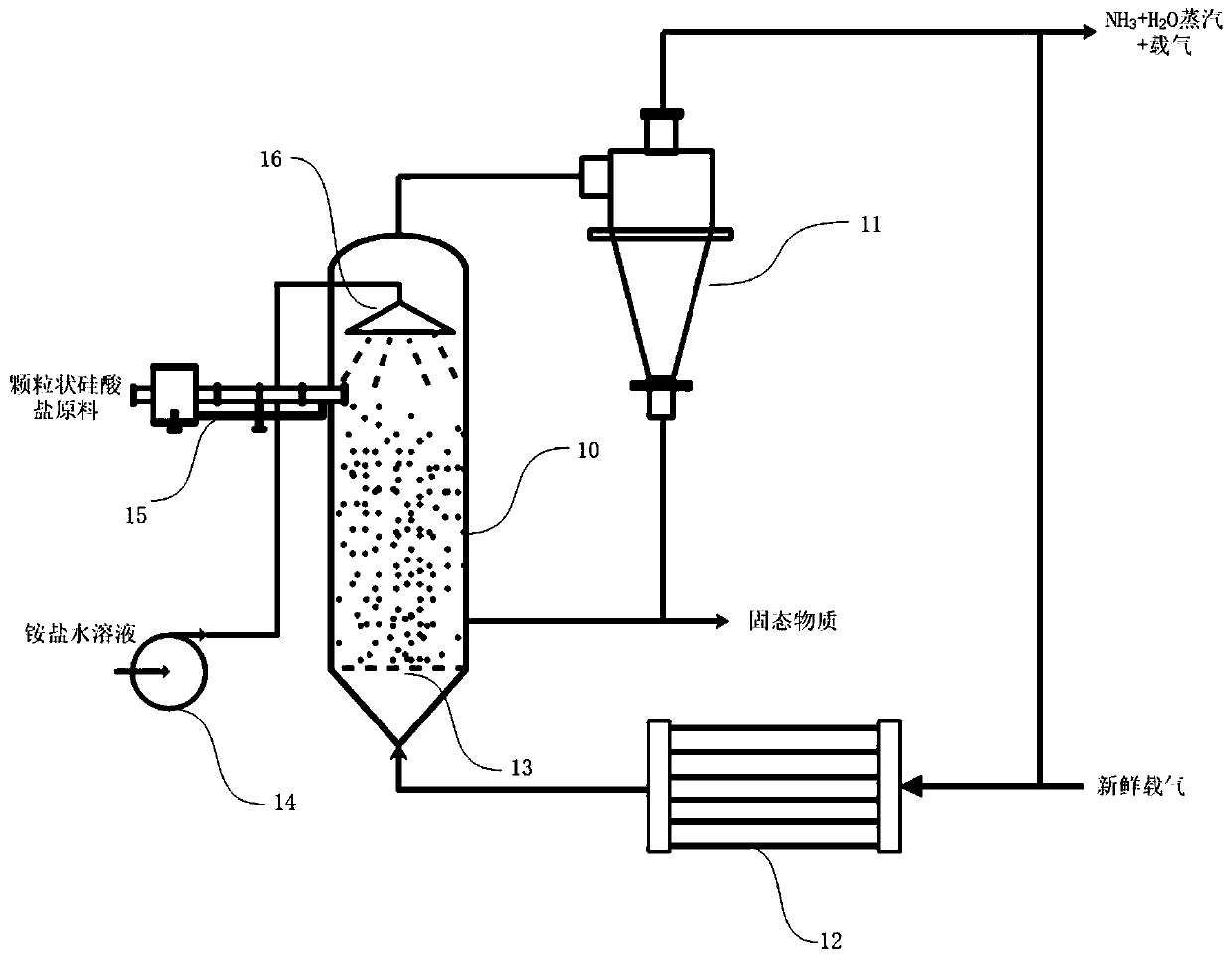
[0041] figure 2It is a schematic diagram of the preparation of ammonia using a fluidized bed in Example 1 of the present invention. A certain amount of granular silicate raw material enters the fluidized bed reactor 10 through the solid feed device 15, and the ammonium salt solution passes through the high-pressure liquefaction pump 14 and the nozzle. Under the action of 16, it reacts with silicate particles in the form of droplets to generate solid matter and ammonia gas. The solid matter is extracted, and a part of it is recycled to mix with fresh silicate particles. Ammonia and water vapor escape into the air with the carrier gas. Solid separation device 11, the separated gas phase containing ammonia is partly drawn out, part of which is recycled to be mixed with fresh carrier gas, preheated by preheater 12, and then enters moving bed reactor 10 through gas distributor 13.
[0042] As a specific example, the total volume of the fluidized bed reactor used in this example is...
Embodiment 2
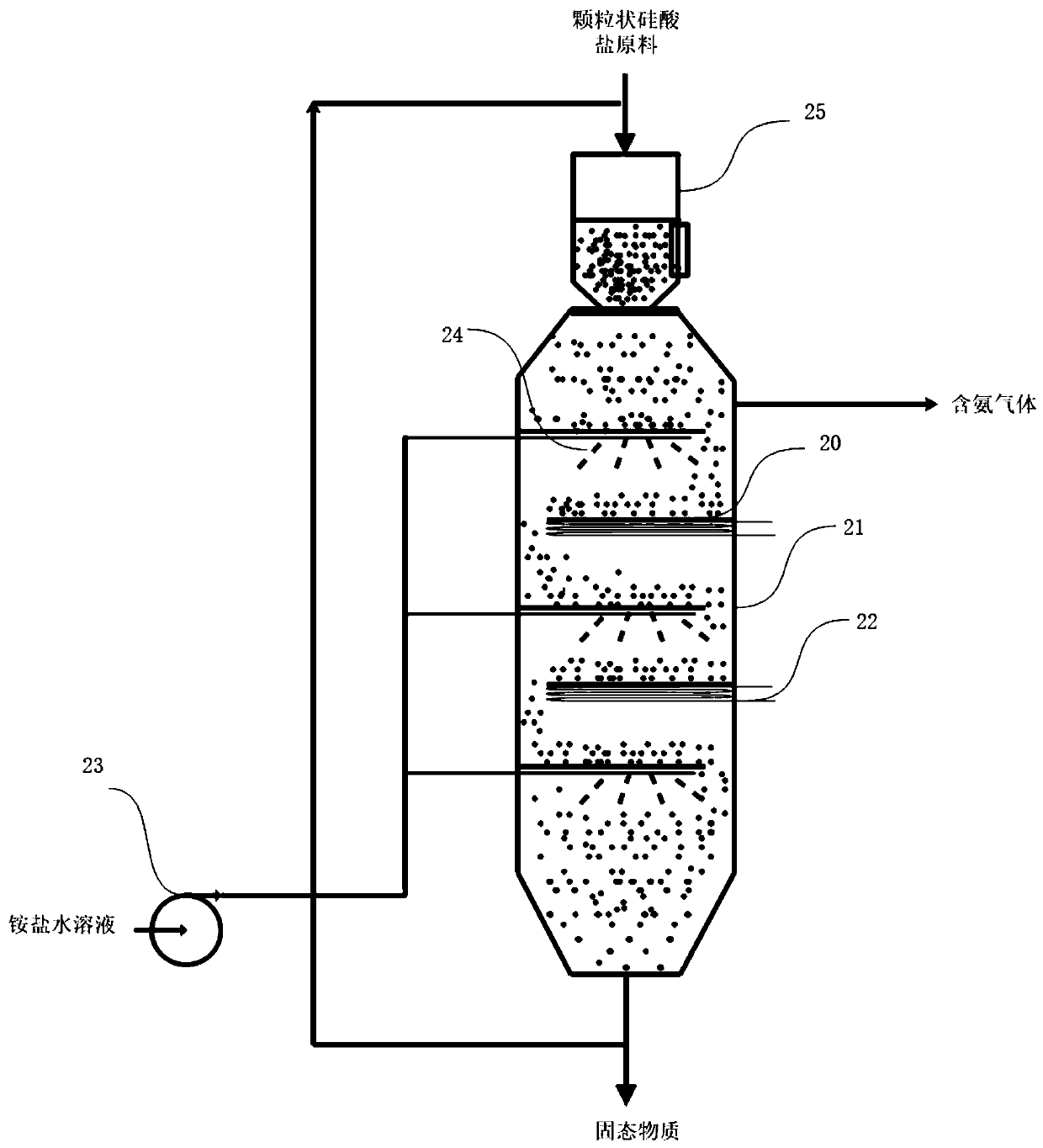
[0047] image 3 It is a schematic diagram of using a moving bed to prepare ammonia in Example 2. A certain amount of granular silicate raw material enters the moving bed reactor 21 through the solid feed device 25, and moves in a broken line under the action of the baffle plate 20. The high temperature medium Heat is supplied to the system through the heating coil 22, and the aqueous ammonium salt solution contacts and reacts with the silicate particles in the form of droplets under the action of the high-pressure liquefaction pump 23 and the nozzle 24 to generate solid matter and ammonia gas, and the ammonia gas is extracted, and the solid matter is collected out, partly recycled to mix with fresh silicate granules.
[0048] As a specific example, the total volume of the moving bed reactor used in this example is 10L, and the aspect ratio is 20:1, that is, the diameter of the reactor is 0.086 meters, and the height is 1.72 meters. Atomizing nozzles and heating coils (not all...
Embodiment 3
[0053] The equipment and process used in this example are the same as in Example 1, except that this example uses wollastonite with a calcium silicate content of 85% as solid particles, the average particle size of which is 0.8 mm, and ammonium chloride is used as the ammonium salt. The molar ratio of ammonium chloride to calcium silicate is 1:2, air is used as the carrier gas, and the operation is at normal pressure. The air is preheated to 450°C in the preheater, and the hot air volume is 15.4m3 / h (under standard conditions). The working temperature is controlled at 300°C, the temperature of the ammonium chloride solution is 90°C, the mass fraction is 40.8%, the spraying rate is 0.3kg / h, and the circulation rate of 50% of the exhaust gas at the outlet is adjusted. The ratio of the amount used is 1:4 (mass ratio), and the additional amount of wollastonite particles is 0.14kg / h. The reaction results are shown in Table 3:
[0054] Table 3 The results of the reaction between amm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com