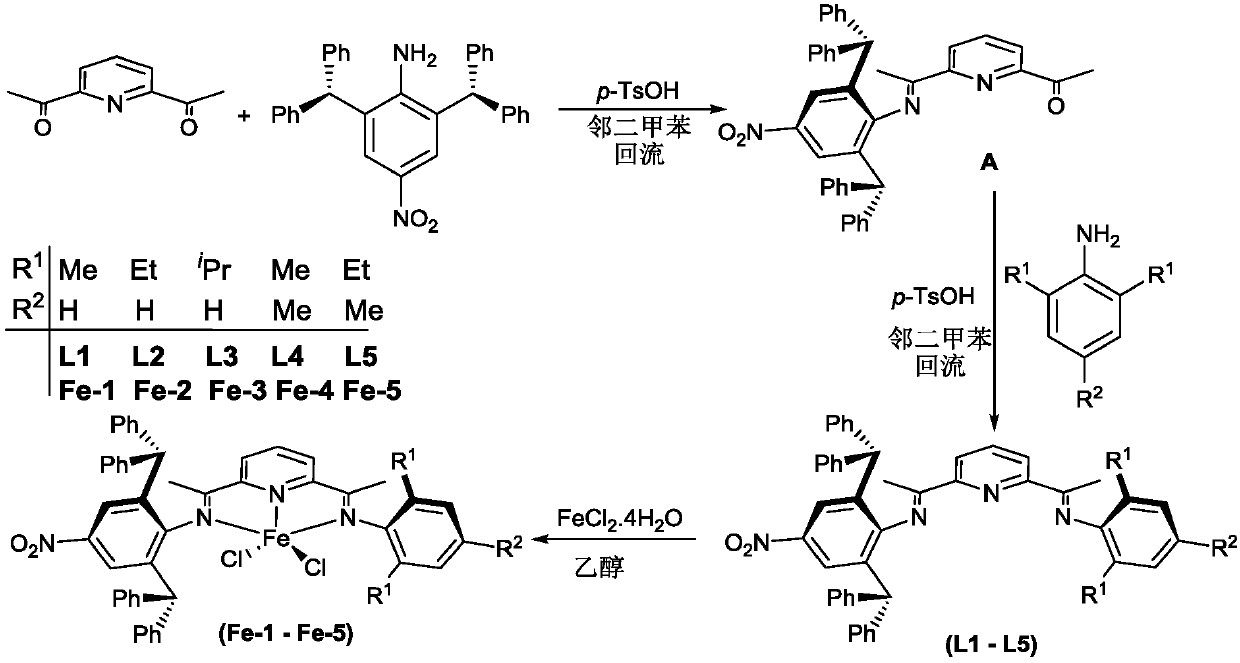
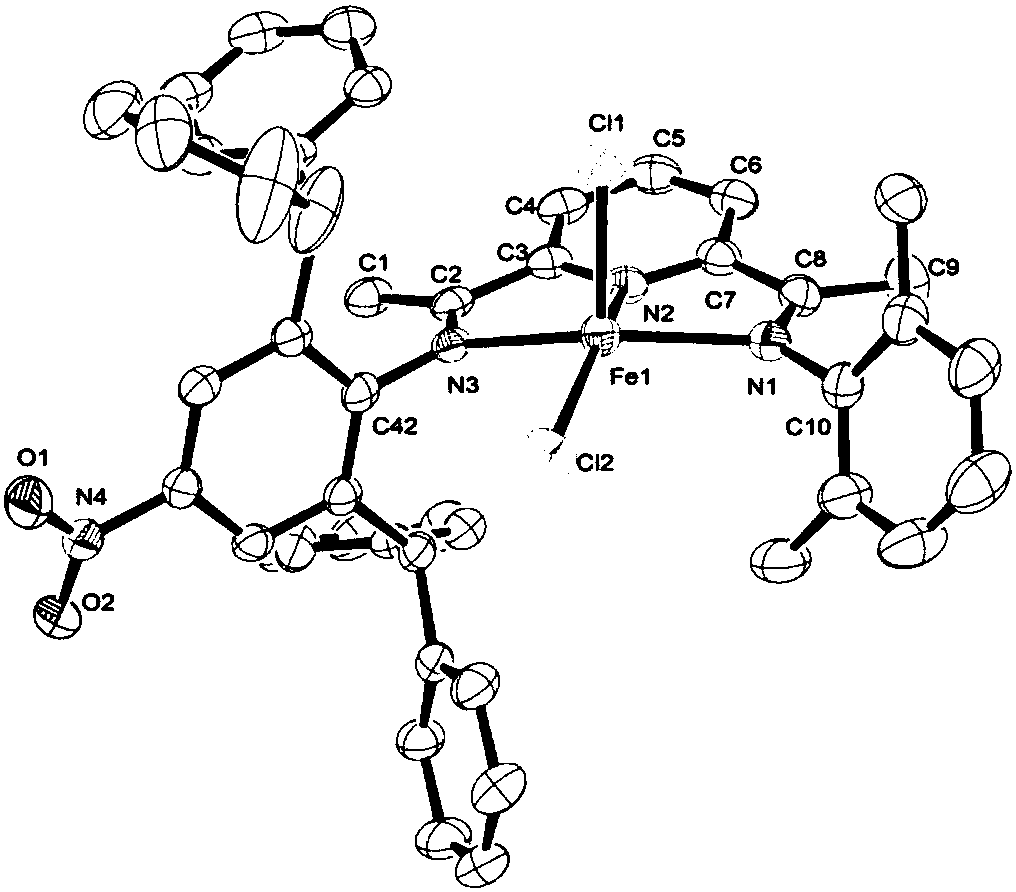
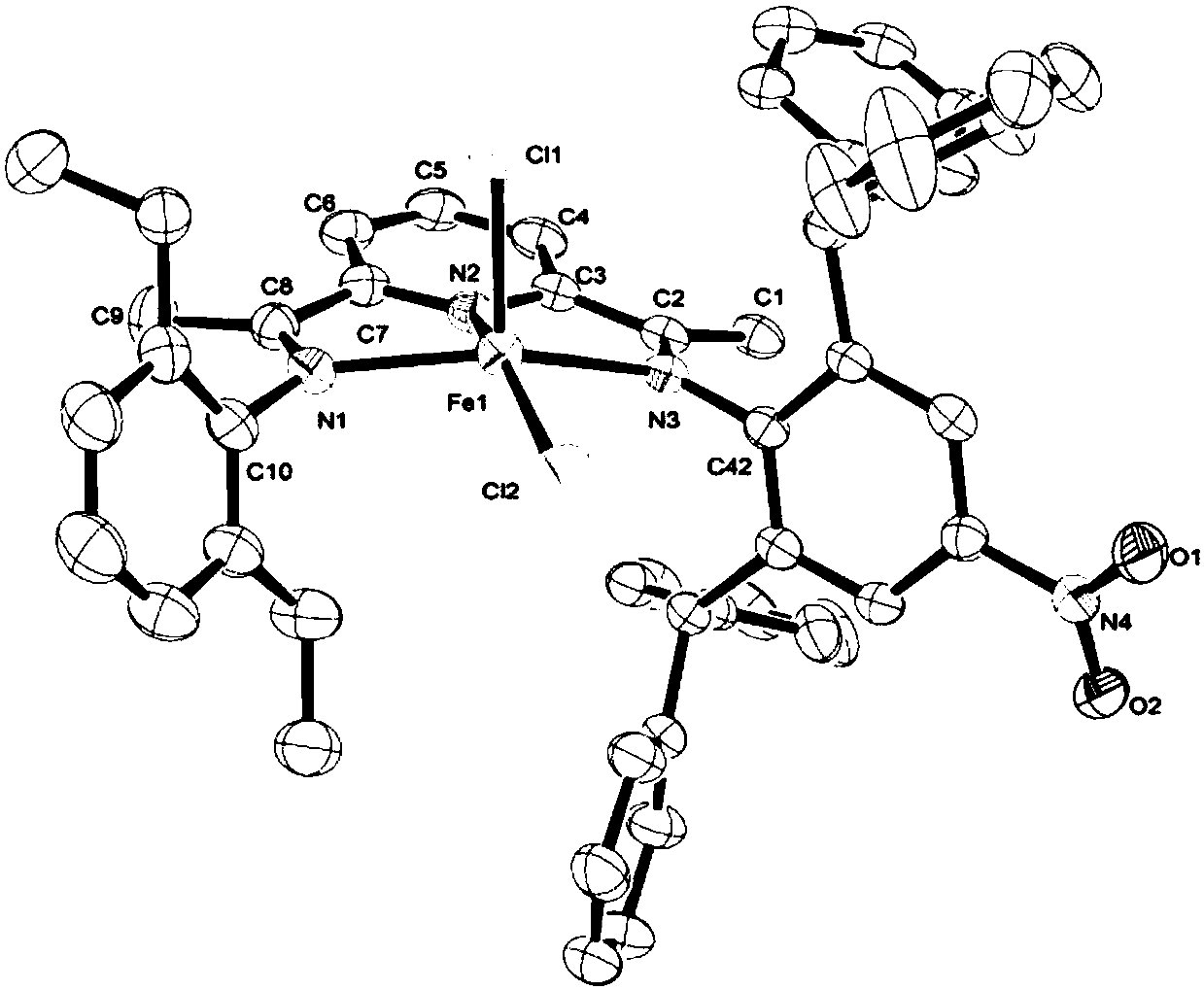
Asymmetric diimine pyridine complex with nitro-enhanced thermal stability and catalytic activity as well as preparation method and application of asymmetric diimine pyridine complex
A technology of complexes and compounds, applied in chemical instruments and methods, iron organic compounds, cobalt organic compounds, etc., can solve the problems of poor thermal stability and reduced catalyst activity of late transition metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1. Preparation of 2-acetyl-6 (1-(2,6-benzhydryl-4-nitro-anilino) ethyl) pyridine (A) shown in the following formula
[0095] Weigh 4.08g (25mmol) of 2,6-diacetylpyridine and 11.77g (25mmol) of 2,6-benzhydryl-4-nitro-aniline into the reaction flask, and add o-xylene to the reaction flask About 160mL, heat the solution to a cool yellow color, indicating that the two reactants have been fully dissolved, and then add a catalytic amount of p-toluenesulfonic acid to the reaction system. After 12 h of stirring at reflux temperature, the reaction mixture was filtered under heating, and all volatiles were evaporated under reduced pressure. Then, the unprocessed product obtained was subjected to column chromatography through a silica gel column, and was eluted with a mixed solvent of sherwood oil and ethyl acetate as an eluent (25 / 1), and the solvent was removed to obtain 5.39 g of a light yellow powder. That is A, 2-acetyl-6(1-(2,6-benzhydryl-4-nitro-anilino)ethyl)pyrid...
Embodiment 2
[0102] Example 2. Preparation of 2-(1-(2,6-benzhydryl-4-nitro-anilino) ethyl)-6(1-(2,6-dimethyl- Anilino)ethyl)pyridine (ligand L1)
[0103] Weigh 2.16g (3.50mmol) 2-acetyl-6(1-(2,6-benzhydryl-4-nitro-anilino) ethyl)pyridine, add it to the reaction flask, and add Add a catalytic equivalent of p-toluenesulfonic acid, and add about 40 mL of o-xylene solvent to form a solution containing A. Dissolve 0.45 g (3.75 mmol) of 2,6-dimethylaniline in o-xylene solution dropwise into the reactor solution containing A. The reaction mixture was heated to reflux for 12h. Cool to room temperature and evaporate volatiles in vacuo. Then, the obtained unprocessed residual solid was eluted through silica gel column chromatography (200:1 (v / v) with a mixed solvent of petroleum ether and ethyl acetate as eluent), and the solvent was removed to obtain 0.75g The light yellow powder is the ligand L1, 2-(1-(2,6-benzhydryl-4-nitro-anilino)ethyl)-6(1-(2,6-dimethyl -anilino)ethyl)pyridine, yield: 30%...
Embodiment 3
[0110] Example 3. Preparation of 2-(1-(2,6-benzhydryl-4-nitro-anilino) ethyl)-6(1-(2,6-diethyl- Anilino)ethyl)pyridine (ligand L2)
[0111] Weigh 2.16g (3.50mmol) 2-acetyl-6(1-(2,6-benzhydryl-4-nitro-anilino) ethyl)pyridine, add it to the reaction flask, and add Add a catalytic equivalent of p-toluenesulfonic acid, and add about 40 mL of o-xylene solvent to form a solution containing A. The o-xylene solution containing 0.56 g (3.75 mmol) of 2,6-diethylaniline was dropped into the reactor solution containing A. The reaction mixture was heated to reflux for 12h. Cool to room temperature and evaporate volatiles in vacuo. Then, the unprocessed residual solid obtained was subjected to column chromatography on a silica gel column (using a mixed solvent of petroleum ether and ethyl acetate 200:1 (v / v) as eluent) to elute, and the solvent was removed to obtain 1.07g The light yellow powder is the ligand L2, 2-(1-(2,6-benzhydryl-4-nitro-anilino)ethyl)-6(1-(2,6-diethyl) -anilino)et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polymerization activity | aaaaa | aaaaa |
| Polymer molecular weight | aaaaa | aaaaa |
| Polymer molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com