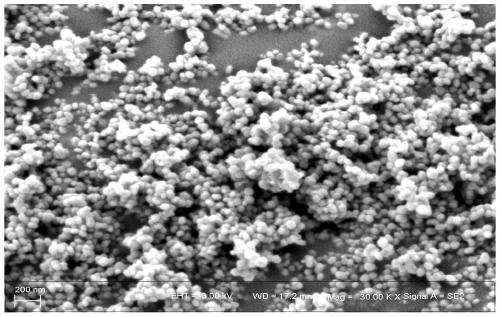
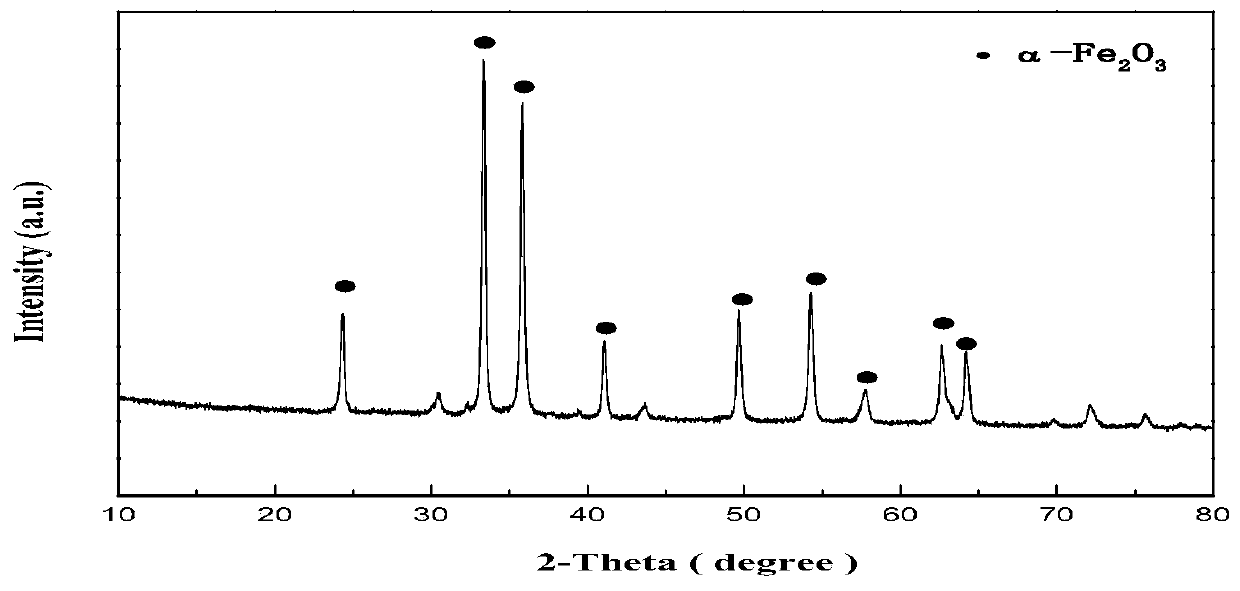
Method for preparing ethylbenzene dehydrogenation catalyst by utilizing spherical nanometer alpha-ferric oxide as iron source
An ethylbenzene dehydrogenation and catalyst technology, which is applied in metal/metal oxide/metal hydroxide catalysts, carbon compound catalysts, physical/chemical process catalysts, etc., can solve the problems of low stability and low catalyst activity, and achieves Improve stability, high activity, increase the effect of dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Mix 30g of ferric nitrate with deionized water, stir and fully dissolve to make a 1mol / L solution. Then add 200ml of 1mol / L urea and stir for 3h to form a gel. The gel substance was aged for 6 hours and dried at 95° C. for 20 hours to obtain an iron oxide precursor. The iron oxide precursor was calcined at 550 °C for 4 h in a muffle furnace. Obtain nano-iron oxide.
[0041] (2) Dissolve 15 g of cerium nitrate in deionized water and mix with 12 mol / L sodium hydroxide. After magnetic stirring for 30 minutes, the pH was adjusted to 2-6 with nitric acid to form white flocs. Put the solution into a polytetrafluoroethylene liner and keep it warm at 160°C for 18h. After hydrothermal pressure treatment, the sample was separated by centrifugation, and then washed several times with deionized water and absolute ethanol solution respectively. Finally, the washed samples were dried in a drying oven at 60 °C for 24 h.
[0042] (3) 67.1g of iron oxide powder and 7.5g of cer...
Embodiment 2
[0045] (1) Mix 30g of ferric nitrate with deionized water, stir and fully dissolve to make a 2mol / L solution. Then add 165ml of 1mol / L urea and stir for 3h to form a gel. The gel substance was aged for 6 hours and dried at 95° C. for 20 hours to obtain an iron oxide precursor. The iron oxide precursor was calcined at 550 °C for 4 h in a muffle furnace. Obtain nano-iron oxide.
[0046] (2) Dissolve 15 g of cerium nitrate in deionized water, and mix with 16 mol / L sodium hydroxide. After magnetic stirring for 30 minutes, the pH was adjusted to 2-6 with nitric acid to form white flocs. Put the solution into a polytetrafluoroethylene liner and keep it warm at 160°C for 18h. After hydrothermal pressure treatment, the sample was separated by centrifugation, and then washed several times with deionized water and absolute ethanol solution respectively. Finally, the washed samples were dried in a drying oven at 60 °C for 24 h.
[0047] (3) 67.1g of iron oxide powder and 7.5g of ce...
Embodiment 3
[0050](1) Mix 30g of ferric nitrate with deionized water, stir and fully dissolve to make a 1mol / L solution. Then add 140ml of 2mol / L urea and stir for 3h to form a gel. The gel substance was aged for 6 hours and dried at 95° C. for 20 hours to obtain an iron oxide precursor. The iron oxide precursor was calcined at 550 °C for 4 h in a muffle furnace. Obtain nano-iron oxide.
[0051] (2) Dissolve 15 g of cerium nitrate in deionized water and mix it with 18 mol / L sodium hydroxide. After magnetic stirring for 30 minutes, the pH was adjusted to 2-6 with nitric acid to form white flocs. Put the solution into a polytetrafluoroethylene liner and keep it warm at 160°C for 18h. After hydrothermal pressure treatment, the sample was separated by centrifugation, and then washed several times with deionized water and absolute ethanol solution respectively. Finally, the washed samples were dried in a drying oven at 60 °C for 24 h.
[0052] (3) 67.1g of iron oxide powder and 7.5g of c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com