Difunctional sulfur-resistant hydrogenation catalyst and preparation method thereof
A hydrogenation catalyst and dual-function technology, which is applied in the direction of catalyst activation/preparation, hydrogenation to hydrocarbons, molecular sieve catalysts, etc., can solve the problems of affecting the hydrogenation performance of the catalyst, the hydrogen overflow effect is not obvious, and it is not easy to precious metals, etc., to improve Anti-poisoning, high catalytic activity, avoiding coking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 8.8wt% H 2 PtCl 6 Add 51 µL of aqueous solution to 10 mL of distilled water, stir well, add 1 g of H-SSZ-13 carrier, stir at 30 °C for 24 h, vacuum dry at 50 °C with a rotary evaporator, and bake at 350 °C for 4 h.
[0026] After the calcined product was placed in a hydrogen environment at 300° C. for 2 h and reduced, a bifunctional supported catalyst was obtained.
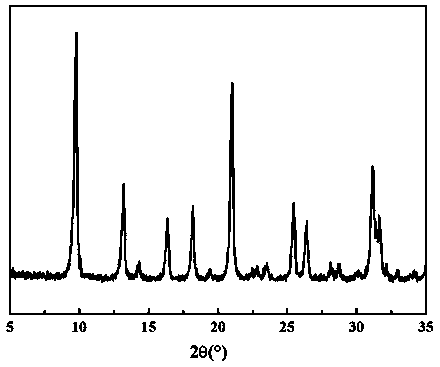
[0027] figure 1 The XRD characterization results of the catalysts prepared above are given. From figure 1 It can be seen that after loading Pt, calcination and reduction, the support structure of the catalyst has not changed, and still shows a strong SSZ-13 characteristic diffraction peak.
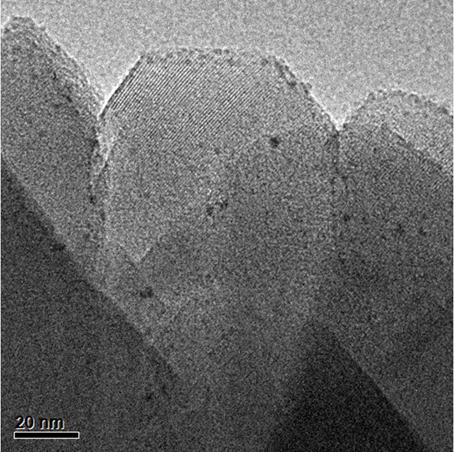
[0028] From figure 2 It can be seen from the TEM image of the catalyst that the Pt supported on the SSZ-13 carrier has a small particle size, about 1.3nm, and is distributed near the crystal edge and close to the orifice.
[0029] Add 0.25 g of naphthalene to the reaction kettle with 10 mL of cyclohexane, di...
Embodiment 2
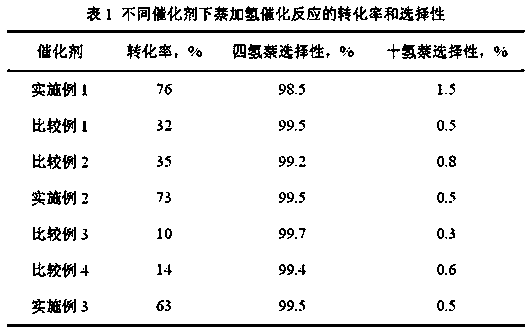
[0038] 50ppm benzothiophene was added to the catalytic reaction system for hydrogenation of naphthalene in Example 1, and other conditions were the same as in Example 1. The concentration of naphthalene, tetrahydronaphthalene and decahydronaphthalene in the reaction solution is detected, and the conversion rate of naphthalene and the selectivity to tetrahydronaphthalene and decahydronaphthalene are calculated. The results are listed in Table 1.
[0039] It can be seen from the catalytic results that after adding 50 ppm benzothiophene, the catalytic activity of the catalyst prepared in Example 1 remains almost unchanged, which proves that the catalyst has good anti-sulfur activity.
Embodiment 3
[0047] Add 100ppm benzothiophene to the catalytic reaction system for hydrogenation of naphthalene in Example 1, and other conditions are the same as in Example 1. The concentration of naphthalene, tetrahydronaphthalene and decahydronaphthalene in the reaction solution is detected, and the conversion rate of naphthalene and the selectivity to tetrahydronaphthalene and decahydronaphthalene are calculated. The results are listed in Table 1.
[0048] As can be seen from the catalytic results in Table 1, compared with Example 1 and Example 2, with the increase of the sulfur content in the reaction system, although the conversion rate of the catalyst prepared with HSSZ-13 as the carrier is somewhat reduced, it still shows Relatively good catalytic activity and sulfur resistance.
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com