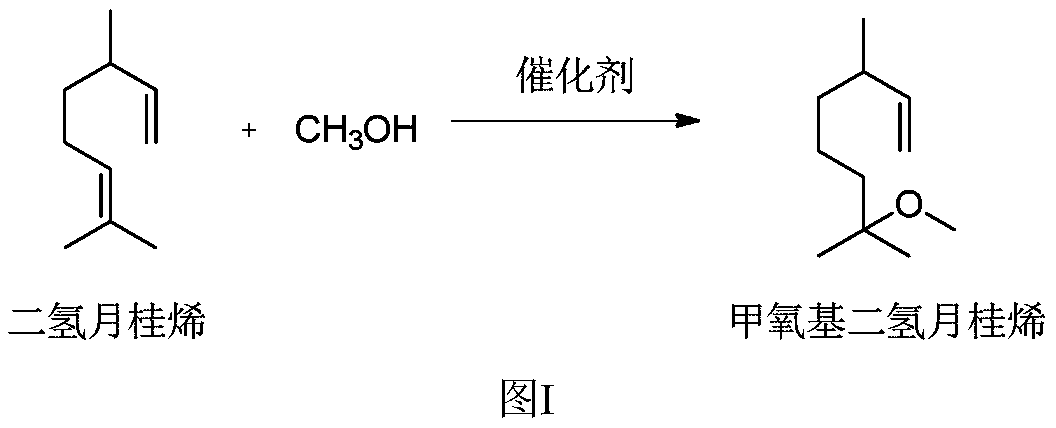
Production method and production system of methoxy elgenol
A production method and technology for sandalwood ether are applied in the field of production methods and production systems of sandalwood ether, which can solve the problems of inability to achieve green atomic chemical synthesis, difficulty in recycling and processing waste catalysts, and restricting the scope of use of products, so as to achieve green The effect of environmentally friendly production, green production process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
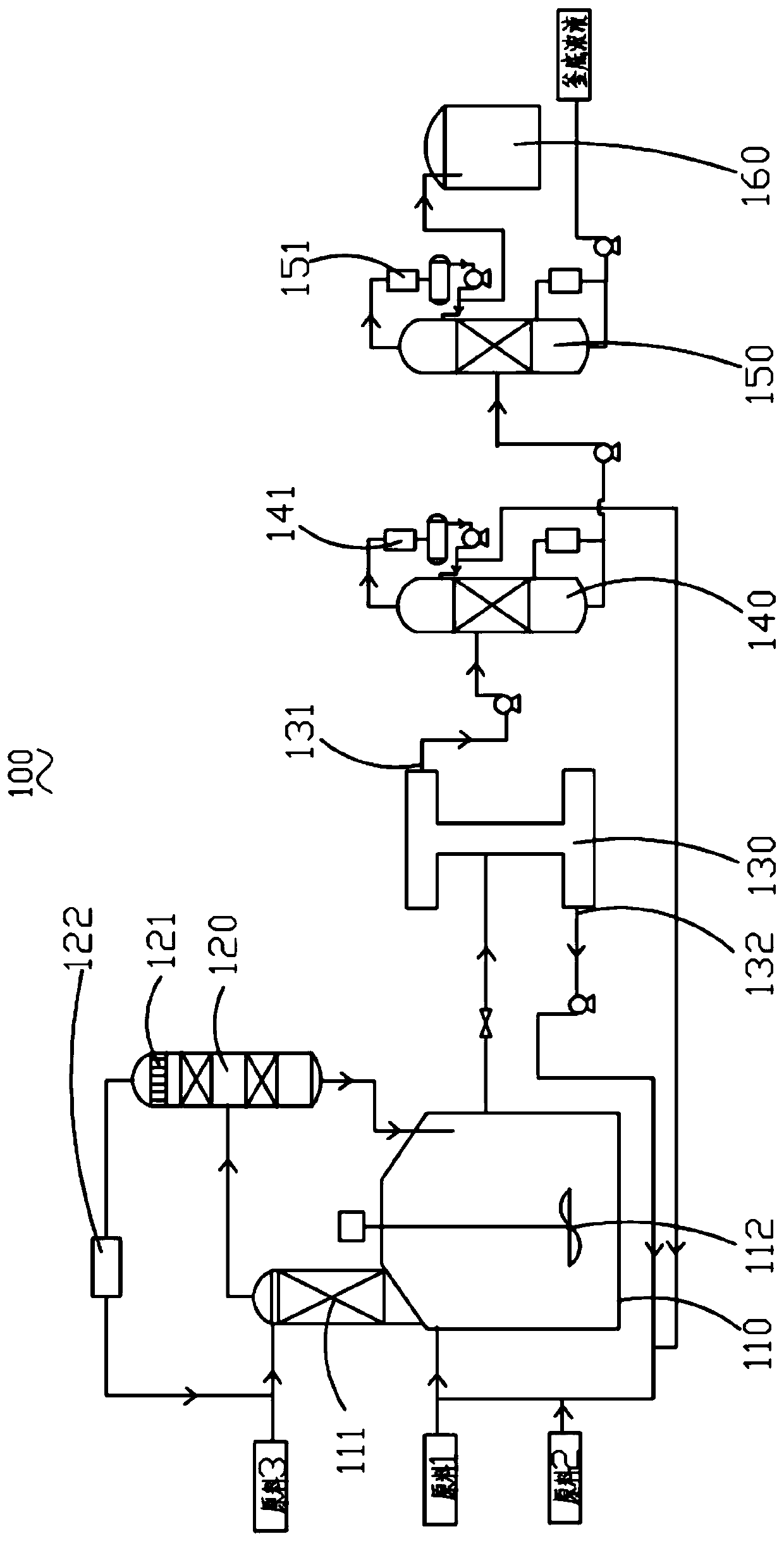
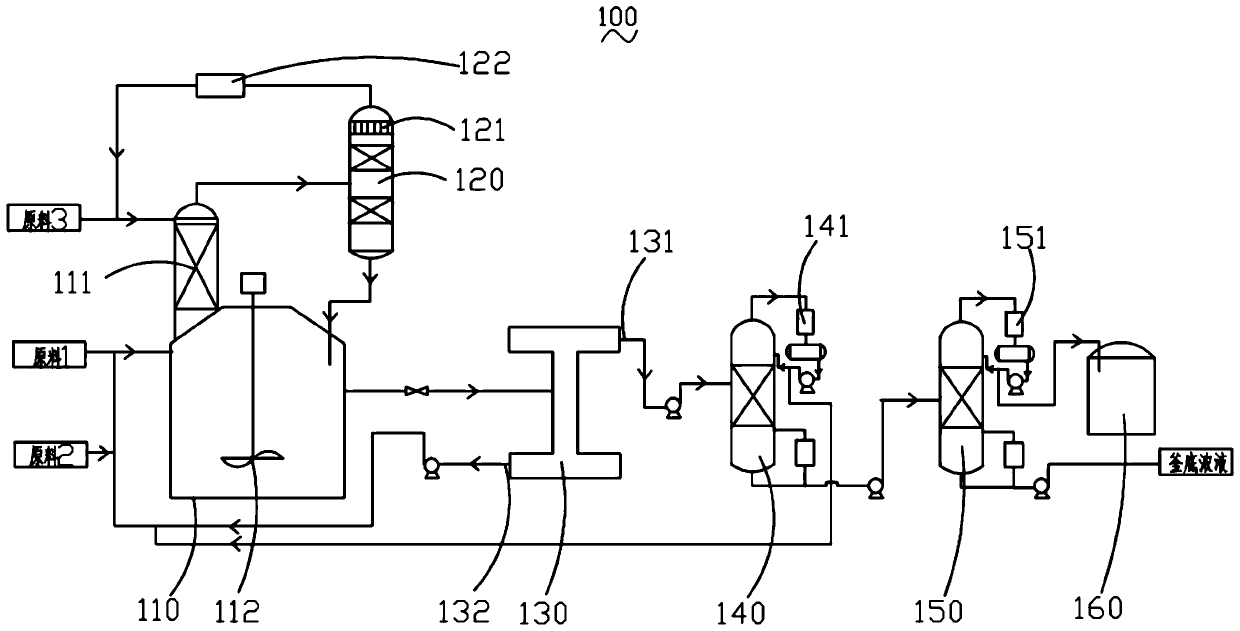
[0040] Specifically, the preparation method of the modified strong acidic cation exchange resin comprises the following steps: using hydrochloric acid with a concentration of 0.1mol / L to 6mol / L to soak the strong acidic cation exchange resin to cause ion exchange; wherein, the amount of hydrochloric acid is 1 to 25 times the weight of the resin, the immersion time is 0.5h to 48h, and the immersion temperature is 10°C to 100°C. Drying the ion-exchanged strongly acidic cation exchange resin at a temperature of 25° C. to 100° C. prepares the modified strongly acidic cation exchange resin.
[0041] Preferably, the strongly acidic cation exchange resin is a styrene-based strongly acidic cation exchange resin, a macroporous cation exchange resin or a zeolite catalyst, specifically, it may be Amberlyst 35 cation exchange resin, NKC-9 cation exchange resin, D72 One or more of cation exchange resin, HZSM-5, mordenite, etc. are used in combination.
[0042] As preferably, the concentra...
specific Embodiment approach 1
[0082] Put the styrene-based strongly acidic cation exchange resin into a 0.1mol / L hydrochloric acid solution, soak it at room temperature for 48 hours at a liquid-to-solid mass ratio of 1:1, and then filter the resin. Chloride ions are entrained (tested by silver nitrate solution), and then dried at 100°C for 48 hours to obtain a modified strongly acidic cation exchange resin, which is ready for use.
specific Embodiment approach 2
[0084] Put the styrene-based strongly acidic cation exchange resin into a 0.5mol / L hydrochloric acid solution, soak it at 10°C for 24 hours at a liquid-solid mass ratio of 10:1, and then filter the resin, then wash the resin with deionized water until there is no entrained chloride ion (tested by silver nitrate solution), and then dried at 60°C for 48 hours to obtain a modified strongly acidic cation exchange resin, which is ready for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com