A kind of detection method of methyl 5-isoquinolinesulfonate and ethyl 5-isoquinolinesulfonate in fasudil hydrochloride
A technology of methyl isoquinoline sulfonate and ethyl isoquinoline sulfonate, which is applied in the detection field of some impurities, can solve the problems such as difficulty in identifying the content of 5-isoquinoline sulfonic acid, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
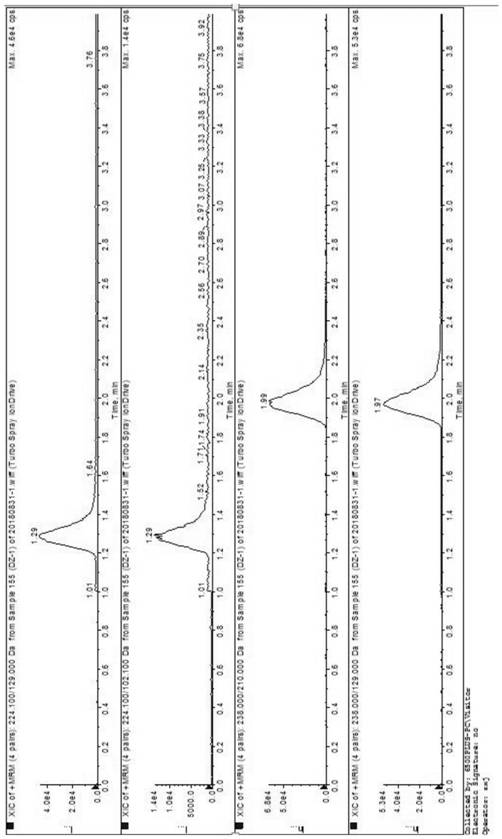
[0029] Embodiment 1 specificity experiment
[0030] (1) Blank solution: Take methanol as the blank solution;
[0031] (2) Preparation of the test solution: Accurately weigh about 12.5 mg of the sample of fasudil hydrochloride, put it in a 10 mL measuring bottle, add methanol and dilute to the mark;
[0032] (3) Preparation of the control solution: Accurately weigh about 10 mg each of methyl 5-isoquinolinesulfonate and ethyl 5-isoquinolinesulfonate, and quantitatively dilute with methanol to make 5-isoquinolinesulfonate containing 5-isoquinolinesulfonate per 1 mL A solution of about 5ng each of methyl ester and ethyl 5-isoquinolinesulfonate;
[0033] (4) Preparation of standard-added test solution: Accurately weigh about 12.5 mg of Fasudil hydrochloride sample, put it in a 10 mL measuring bottle, add standard solution and dilute to the mark (the content of each reference substance is about 5 ng / mL) .
[0034] (5) Precisely measure 1 µL each of the blank solvent, the test sol...
Embodiment 2
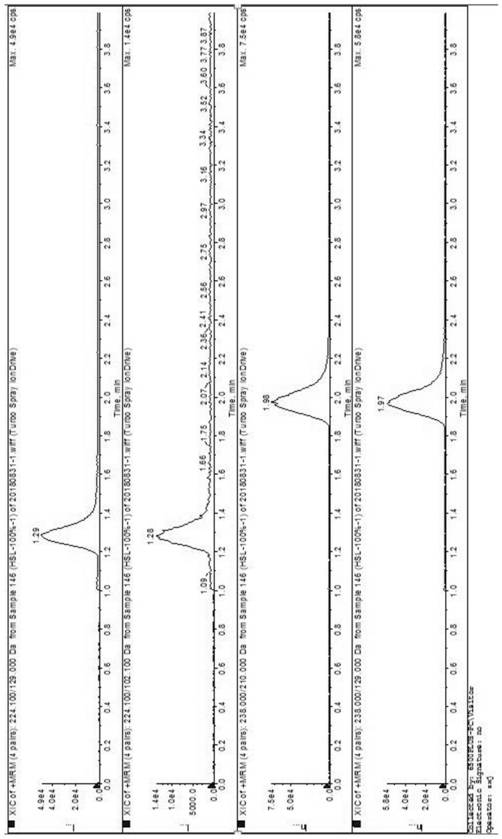
[0038] Embodiment 2 precision experiment
[0039] Preparation of the control solution: Accurately weigh about 10 mg (9.81 mg and 10.23 mg) of methyl 5-isoquinolinesulfonate and ethyl 5-isoquinolinesulfonate, and quantitatively dilute with methanol to make 5- A solution of about 5 ng each of methyl isoquinolinesulfonate and ethyl 5-isoquinolinesulfonate;
[0040] Precisely measure 1 µL of the reference solution, inject it into the liquid mass spectrometer, record the chromatogram, inject 6 consecutive samples, and count the RSD values of the retention time and peak area for 6 times to investigate the precision of the method. The test results are shown below surface;
[0041] Table 1 Results of the applicability test of the verification system for 5-isoquinolinesulfonate methyl ester and 5-isoquinolinesulfonate ethyl ester
[0042]
[0043] Verification requirements: RSD of retention time and peak area shall not exceed 5.0%.
[0044] Conclusion: From the above test resul...
Embodiment 3
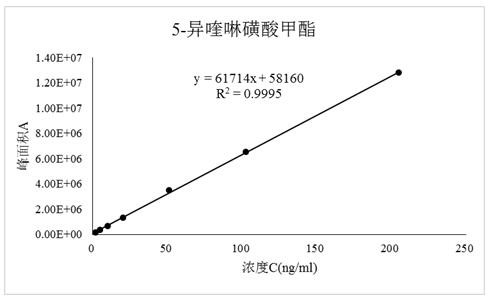
[0045] Embodiment 3 linear relationship experiment
[0046]Preparation of linear solution: Accurately weigh 10.38 mg and 9.56 mg of methyl 5-isoquinoline sulfonate and ethyl 5-isoquinoline sulfonate in the same volumetric flask, and quantitatively dilute with methanol so that each 1 mL contains about 200 ng, A series of solutions of 100ng, 50ng, 20ng, 10ng, 5ng, and 2ng were used as linear solutions of 4000%, 2000%, 1000%, 400%, 200%, 100%, and 40% concentration of the control solution.
[0047] Precisely measure 1 µL of each linear solution, inject it into the liquid mass spectrometer respectively, record the chromatogram, and take the concentration as the abscissa ( X ), the peak area is the ordinate ( Y ) for linear regression. The test results are shown in the table below.
[0048] Table 2 5-isoquinolinesulfonate methyl ester, 5-isoquinolinesulfonate ethyl ester inspection method validation linear relationship test results
[0049]
[0050] Verification requirements...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com