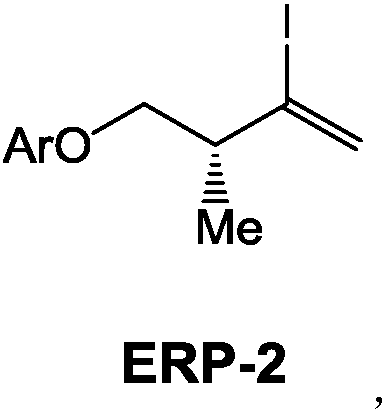
(3R)-2-iodo-4-benzyloxy-3-methyl-1-ene compound as well as preparation method and application thereof
A compound, ERP-3 technology, applied in the field of medicinal chemistry, can solve the problems of inconvenient industrial production, high catalyst cost, high production cost, etc., achieve the effects of improving yield and quality, simple preparation method, and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
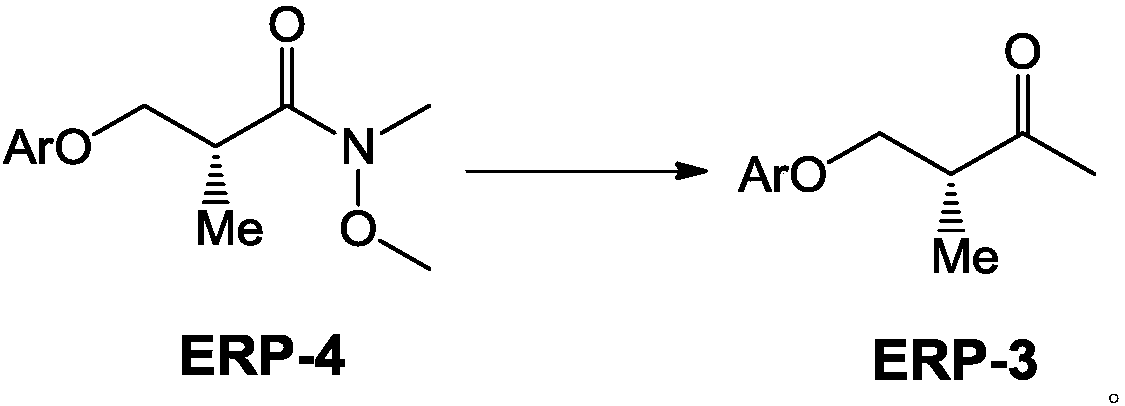
[0044] Embodiment 1: the preparation method of compound ERP-4A:
[0045]
[0046] Add 7Kg of compound ERP-5A into 70L of dichloromethane solution, stir to dissolve, and when the temperature of the reactor is lowered to 10°C, add 7Kg of CDI solid in batches, keep the reactor at 10-15°C, and stir for about 1 hour. Then cool down to 0°C, f add 3.58Kg of N,O-dimethylhydroxylamine hydrochloride in batches, then slowly add 7.3kg of triethylamine, after the dropwise addition, rise to room temperature at 20°C to react, and TCL detects that the raw materials have reacted completely Finally, 30L of water was added to the system, stirred, separated, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and then purified with a silica gel column, eluent (PE / EA=10 / 1), to obtain ERP- 4A, yield 89%, HPLC: 98%. LCMS; MW = 237.30.
Embodiment 2
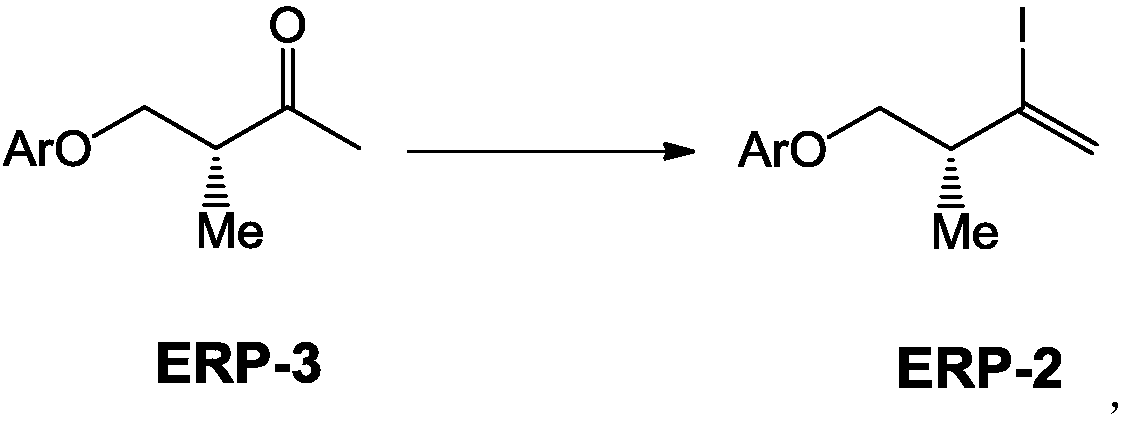
[0047] Embodiment 2: the preparation method of compound ERP-3A:
[0048]
[0049] In a 100L reaction kettle, under nitrogen protection, add 7.3Kg compound ERP-3A and 50L dry THF solution, stir and dissolve, then cool down the reaction kettle, and when the internal temperature drops to -5°C, slowly add 13L dropwise at a concentration of 3mol / L of methylmagnesium chloride, keeping the temperature below 0°C, then insulated and stirred for reaction. TLC detects that the raw materials have reacted completely, add 30L saturated ammonium chloride, stir, let stand to separate layers, extract the aqueous layer with 600ml*2 EA, wash the combined organic phase with saturated brine, dry over anhydrous sodium sulfate, and then use a silica gel column Purification, eluate (PE / EA=10 / 1), yield compound ERP-3A, yield 95%, HPLC: 98%. LCMS; MW = 192.26.
Embodiment 3
[0050] Embodiment 3: the preparation of compound ERP-2A:
[0051]
[0052] (1) In a 50L reaction kettle, under the protection of nitrogen, add 5.5Kg compound ERP-3A and 15L of 80% hydrazine hydrate in batches, then heat up to 80°C and reflux for 2h, TLC detects that the raw materials have reacted completely; cool down the reaction kettle The layers were separated, the aqueous phase was back-extracted once with THF, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and filtered for later use;
[0053] (2) In a 100L reaction kettle, protected by nitrogen, add 20L of THF solvent and stir, replace with nitrogen, then add 13.6Kg iodine in batches, cool the reaction kettle to -5°C, and slowly add 15kg of tetramethylguanidine dropwise , and stirred for 30min, then slowly added dropwise the THF mixed solution of step (1), after the dropwise addition, the reaction kettle was raised to room temperature and stirred for 2h, after the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com