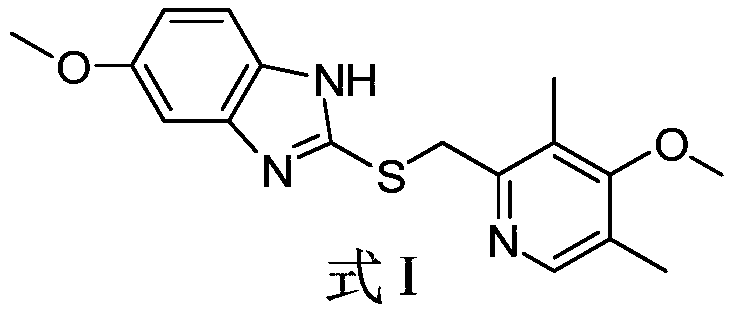
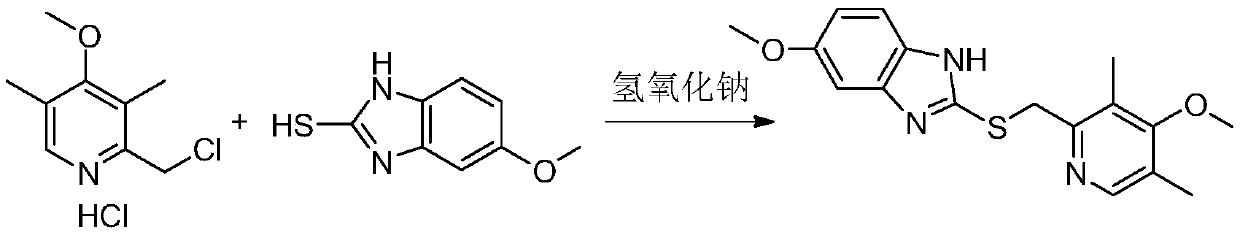
Preparation method of esomeprazole sodium thioether intermediate
An intermediate, the technology of azole thioether, applied in the field of pharmaceutical preparation, can solve the problems of inability to remove water, affect the high selectivity of selective oxidation reaction, etc., and achieve the effect of high stability, high selectivity and low water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Mix methanol (48mL), 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (10.0g), 2-mercapto-5-methoxybenzimidazole (8.2g) , Sodium hydroxide (4.8g) was dissolved in purified water (10mL) and added to the reaction flask, heated to 60°C for 1 hour. The temperature was lowered to 25°C, and purified water (40 ml) was added to the reaction solution. Adjust the pH to 9-10 with hydrochloric acid. The reaction solution was extracted with chloroform (50ml×2) and separated. The organic layer was dried with anhydrous sodium sulfate and filtered. Cumene (30ml) was added to the filtrate and concentrated under reduced pressure to a remaining volume of 20mL. Cumene (30 ml) and diethyl ether (100 ml) were added to the residue, and the mixture was stirred and crystallized at 10°C for 8 hours. After filtration, the filter cake was vacuum dried to obtain the omeprazole intermediate (14.2 g). The yield is 96%, and the moisture content is 0.03%.
Embodiment 2
[0038] Add dimethylformamide (48mL), 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (10.0g), 2-mercapto-5-methoxybenzimidazole (8.2g), sodium hydroxide (4.8g) was dissolved in purified water (10mL) and added to the reaction flask, heated to 70°C for 1 hour. The temperature was lowered to 25°C, and purified water (40 ml) was added to the reaction solution. Adjust the pH to 10-11 with hydrochloric acid. The reaction solution was extracted with chloroform (50ml×2) and separated. The organic layer was dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure. During the process, xylene (30ml) was added, and the concentration was continued to the remaining volume of 20mL. Xylene (30 ml) and isopropyl ether (100 ml) were added to the residue, and the mixture was stirred and crystallized at 10°C for 8 hours. After filtration, the filter cake was dried under vacuum to obtain the omeprazole intermediate (14.1 g). The yield is ...
Embodiment 3
[0040] Combine 1,4 dioxane (50 mL), 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride (10.0g), 2-mercapto-5-methoxybenzene The bisimidazole (8.2g) and sodium hydroxide (4.8g) were dissolved in purified water (15mL) and added to the reaction flask, and the temperature was raised to 80°C for 1 hour. The temperature was lowered to 25°C, and purified water (35 ml) was added to the reaction solution. Adjust the pH value to 8-9 with hydrochloric acid. The reaction solution was extracted with dichloromethane (50ml×2) and separated. The organic layer was dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure. After completion, toluene (30ml) was added and the concentration was continued to the remaining volume of 20mL. Toluene (30 ml) and n-heptane (100 ml) were added to the residue, and the mixture was stirred and crystallized at 10°C for 8 hours. After filtration, the filter cake was dried under vacuum to obtain the omeprazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com