Preparation method of alkoxylation intermediates on the basis of diester-sebacate nitroxyl radicals
A technology of sebacic acid diester nitroxide free radicals and nitroxide free radicals, which is applied in the field of preparation of alkoxylated intermediates, can solve the problems of complex process, low yield, and reduced yield, and achieve wide source of raw materials , low reaction temperature, rapid reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
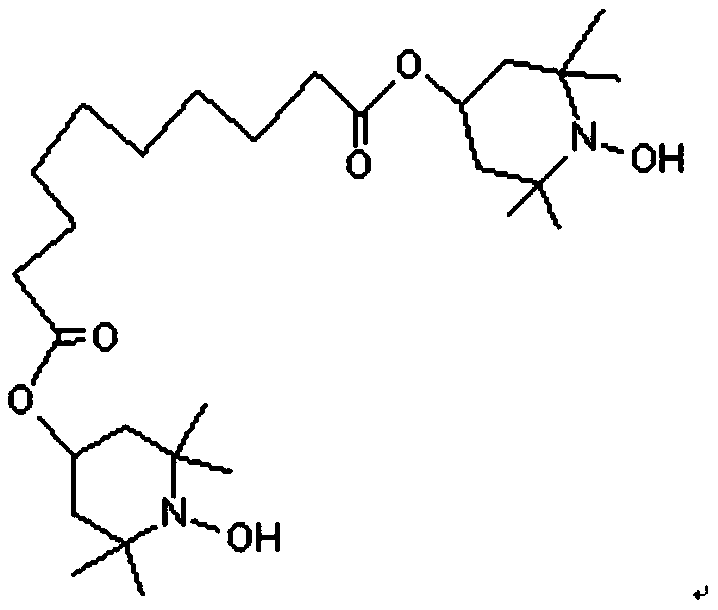
[0028] Put 100g of bis(2,2,6,6-tetramethyl-4-hydroxypiperidine) sebacic acid nitroxide free radicals into the electrolytic reaction bottle, and then add 30g of water, 60g of methanol, and 16g of hydroxide Sodium, 30g formic acid, at 800A / m 3 Electrolytic catalytic oxidation under current density, the anode material is BDD electrode, and the cathode material is titanium plate. After 24 hours of electrolysis, the electrolyte solution is filtered to obtain 97g of alkoxylated intermediate product of sebacic acid diester nitroxide free radical, the content (GC) 97.40%, the yield is about 89%.
[0029]
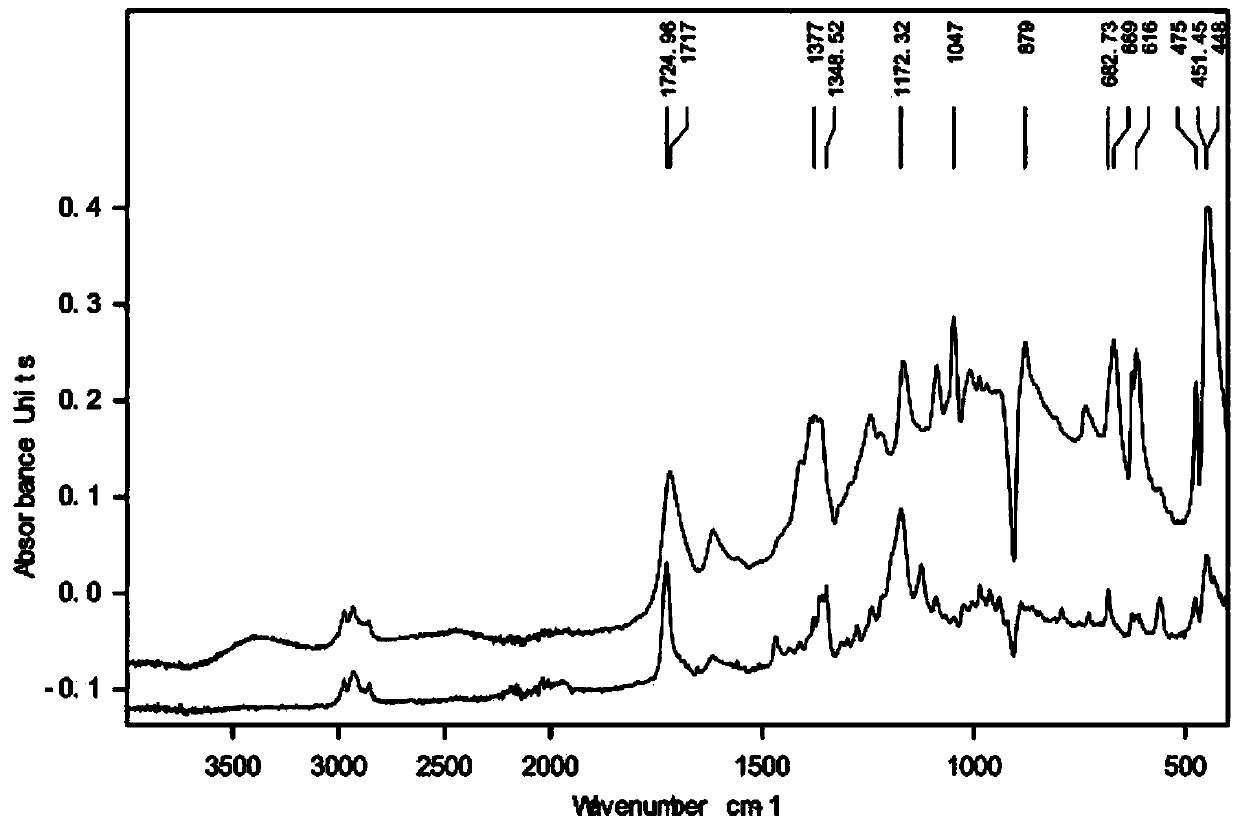
[0030] figure 1 It is the infrared spectrogram of the product prepared under formic acid environment; before electrolysis, there is no characteristic peak of hydroxyl (νOH) in the functional group region, and the infrared peak of the product after electrolysis corresponds to 3405 (νOH) with a characteristic peak of hydroxyl; it shows that in the electrolysis reaction, nitrogen ...
Embodiment 2
[0033] Put 100g of bis(2,2,6,6-tetramethyl-4-hydroxypiperidine) sebacic acid nitroxide free radicals into the electrolytic reaction bottle, and then add 15g of water, 40g of methanol, and 8g of hydroxide Sodium, 30g formic acid, at 300A / m 3 Electrolytic catalytic oxidation under current density, the anode material is BDD electrode, the cathode material is titanium plate, after 24 hours of electrolysis, the electrolyte solution is filtered to obtain 88g of alkoxylated intermediate product of sebacic acid diester nitroxide free radical, the content (GC) 88.35%, the yield is about 82%.
Embodiment 3
[0035] Put 100g of bis(2,2,6,6-tetramethyl-4-hydroxypiperidinyl) sebacic acid nitroxide free radical into the electrolytic reaction bottle, then drop into 45g of water, 50g of methanol, and 6g of hydroxide Potassium, 50g formic acid, at 600A / m 3 Electrolytic catalytic oxidation under current density, the anode material is BDD electrode, and the cathode material is titanium plate. After 24 hours of electrolysis, the electrolyte solution is filtered to obtain 93g of alkoxylated intermediate product of sebacic acid diester nitroxide free radical, the content (GC) 93.25%, the yield is about 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com