Method for hypoxia culture of endometrial stem cell from menstrual blood
An endometrial and stem cell technology, applied in the field of stem cells, can solve the problems of high oxygen concentration and differences in tissue microenvironment, and achieve the effects of strong proliferation and differentiation ability, strong survival ability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Collection of menstrual blood samples from adult women
[0034] (1) Preparation of specimen preservation solution: PBS buffer solution, respectively add gentamicin, 0.2 mg / ml amphotericin, and 1.0 mg / ml EDTA-NA2 at a final concentration of 200 U / ml. Mix well.
[0035] (2) Eight healthy adult female volunteers were recruited 3 days before the menstrual cycle, and about 10ml of their menstrual blood samples were collected, and then the menstrual blood samples were transferred to a 50ml centrifuge tube containing 10ml of specimen preservation solution, and mixed gently. The menstrual blood sample preservation solution was stored at 4°C, and the separation and extraction of endometrial stem cells were completed within 24 hours.
Embodiment 2
[0036] Example 2 Preparation of hypoxic endometrial stem cells
[0037] Take 4 parts of the menstrual blood sample preservation solution in Example 1 and centrifuge at 300 g for 5 min respectively. Collect part of the supernatant and reserve samples for microbial detection, and discard the rest, leaving the precipitate for further operation in a biological safety cabinet.
[0038] For further precipitation, add 20ml of normal saline and mix well, slowly add to the upper layer of 10ml of Ficoll lymphocyte separation medium, add liquid carefully to keep a clear interface between the blood sediment and Ficoll lymphocyte separation medium. Centrifuge at 400g for 20 minutes, carefully suck off the uppermost liquid, and retain the buffy coat cells and endometrial fragments. Wash twice with PBS buffer, then add 5ml of complete medium (low sugar DMEM and F12 are mixed in proportion, then add plasma replacement with a final concentration of 10%, gentamicin 100U / ml, amphotericin 0.1mg / ...
Embodiment 3
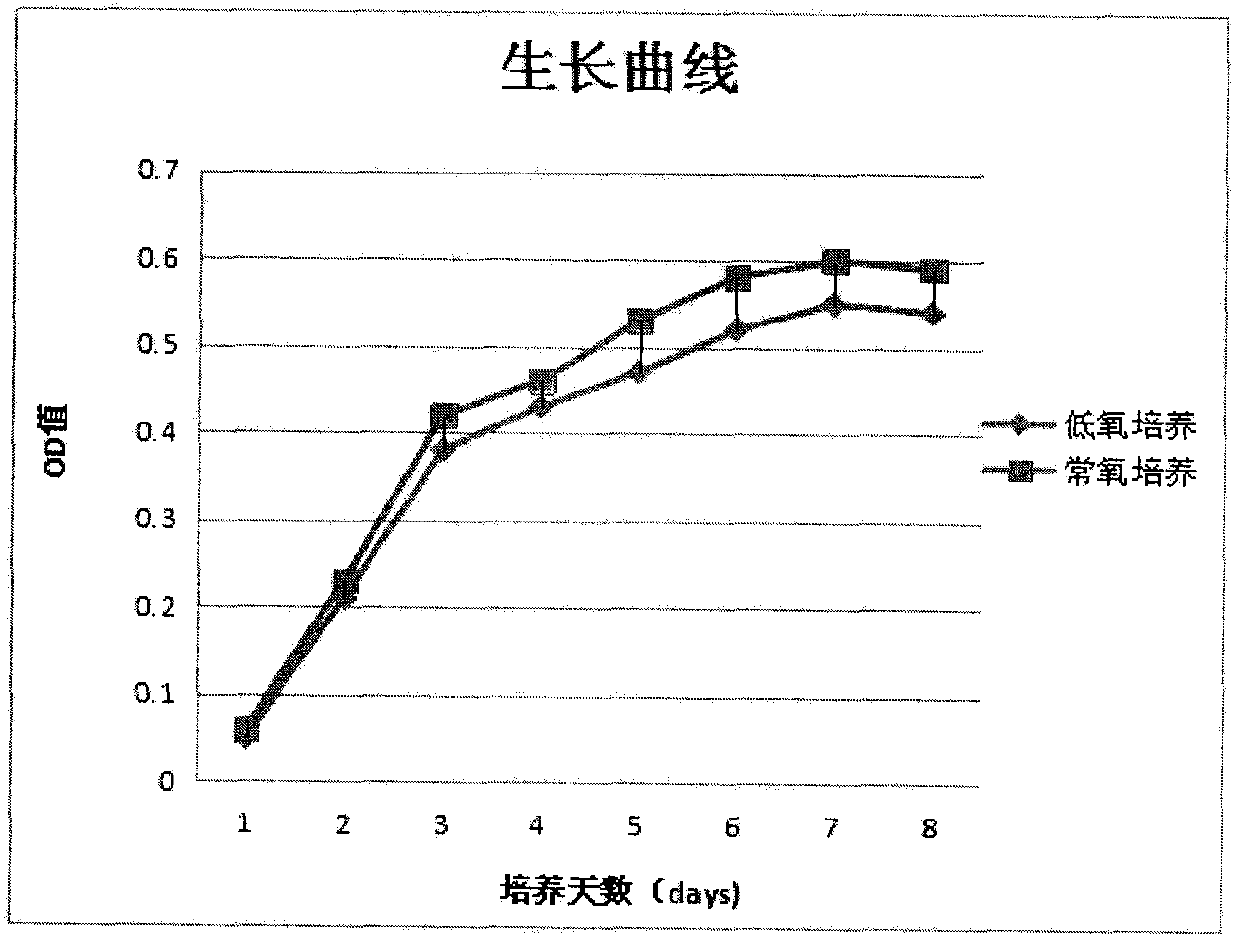
[0040] Example 3 Growth curve of endometrial stem cells derived from menstrual blood
[0041] Detection of growth curve of endometrial stem cells in menstrual blood: culture endometrial stem cells to the third passage in hypoxic carbon dioxide incubator and normal oxygen carbon dioxide incubator, digest cells with 0.25% trypsin, and resuspend with appropriate volume of medium cells, take 100 μl single cell suspension (1×10 3) were seeded in 96-well plates. The cells were placed in the hypoxic incubator and the normoxic incubator for 8 days, and each day was used as a time point and three replicate holes were set up; 10 μl of CCK-8 solution was added to the 96-well plate 4 hours before the measurement; The standard instrument detects the OD value of each well at a wavelength of 490nm, and records the results. Draw the growth curve, see the results figure 1 .
[0042] from figure 1 It can be seen that MenESCs cultured in normoxia and hypoxia both showed a good proliferation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com