A kind of preparation method of gefitinib composite particle
A technology of gefitinib and composite particles, applied in the field of medicine, can solve the problems of high cost, achieve high dissolution rate, improve solubility, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
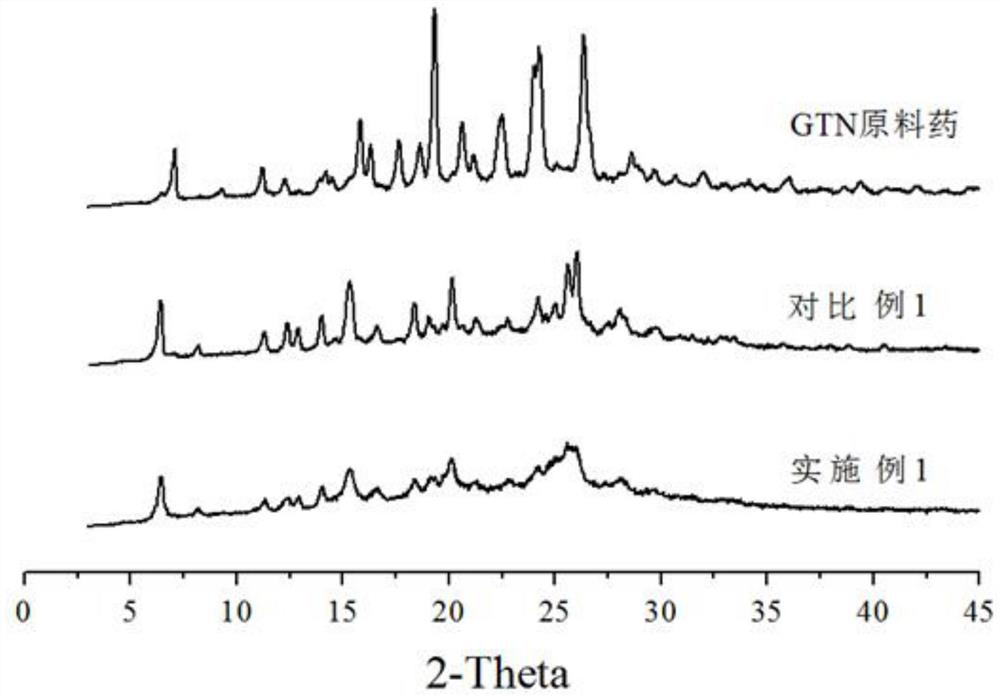
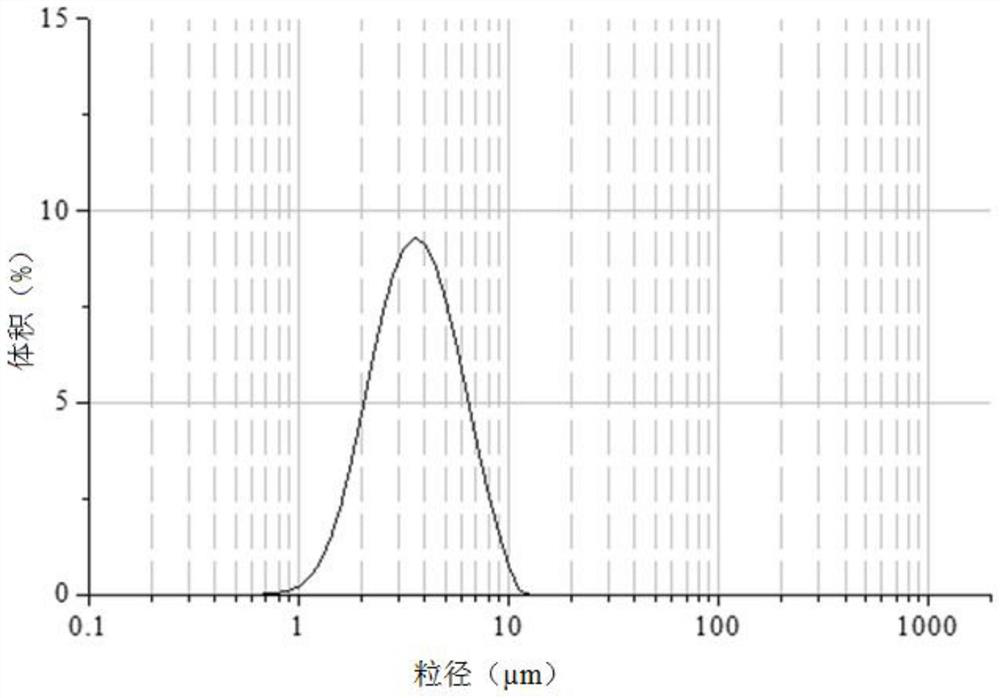
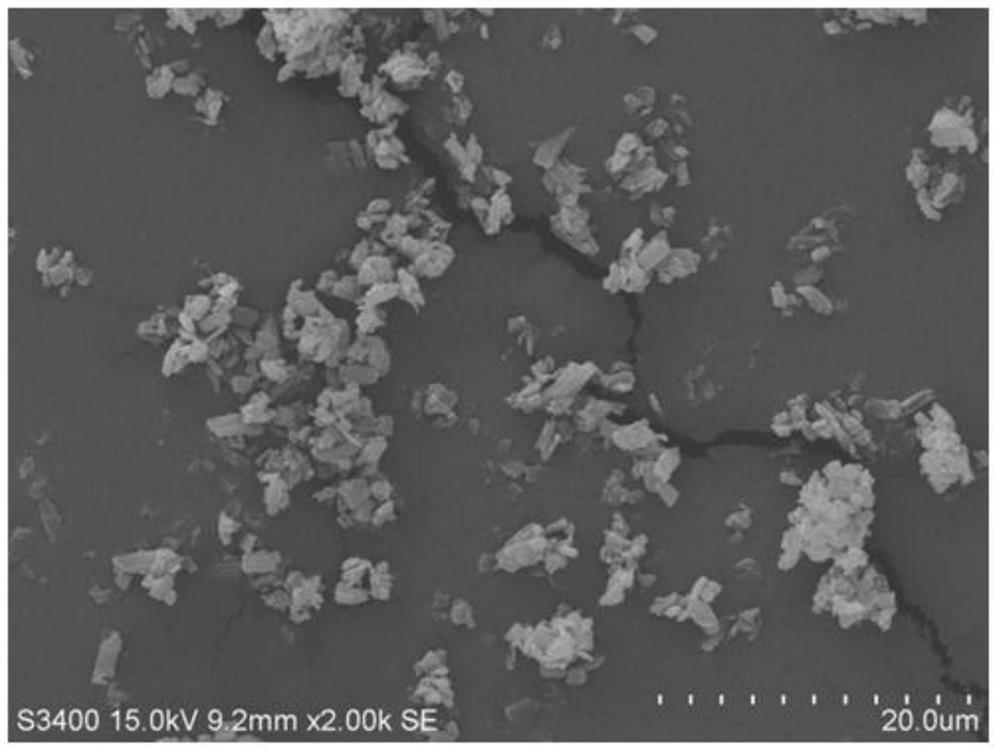
[0038] At room temperature, 100 mg of gefitinib bulk drug was dissolved in 1 mL of dimethyl sulfoxide to obtain a good solvent phase. 300 mg of zein was dissolved in 100 mL of deionized water, and the pH was adjusted to 9 with 0.5 M NaOH solution until the zein was completely dissolved to obtain an aqueous phase. When the temperature is 25°C, inject the good solvent phase into the water phase, stir for 30 minutes, then filter with suction, collect the filter cake, wash with deionized water 90 times the mass of gefitinib, and place the filter cake in vacuum drying after suction filtration Dry in an oven at 60°C for 8 hours to obtain gefitinib composite particles. The particle size is shown in Table 1, and Table 1 is the particle size distribution parameters of the gefitinib composite particles.
[0039] Before collecting the gefitinib composite microparticles, inject the good solvent phase into the aqueous phase and stir the suspension for 30 minutes, and use a Malvern 2000 pa...
Embodiment 2
[0057] Dissolve 99.7mg of gefitinib raw material in 1mL of N,N-dimethylformamide, heat to 45°C and ultrasonically dissolve to obtain a good solvent phase. 501.3 mg of zein was dissolved in 100 mL of deionized water, and the pH was adjusted to 9 with 0.5 M NaOH solution until the zein was completely dissolved to obtain an aqueous phase. When the temperature is 25°C, inject the good solvent phase into the water phase, stir for 30 minutes, then filter with suction, collect the filter cake, wash with deionized water 90 times the mass of gefitinib, and place the filter cake in vacuum drying after suction filtration Dry in an oven at 60°C for 7 hours to obtain gefitinib composite particles.
[0058] Before collecting the gefitinib composite particles, inject the good solvent phase into the water phase and stir the suspension for 30 minutes, and measure the particle size with a Malvern 2000 particle size analyzer. The D90 is 8.287 μm, and X-ray is carried out on the dried particle po...
Embodiment 3
[0060] Under the condition of temperature of 40°C, 102.2mg of gefitinib bulk drug was dissolved in 1mL of dimethyl sulfoxide to obtain a good solvent phase. 300.2 mg of zein was dissolved in 100 mL of deionized water, and the pH was adjusted to 9 with 0.5 M NaOH solution until the zein was completely dissolved to obtain an aqueous phase. When the temperature is 20°C, inject the good solvent phase into the water phase, stir for 30 minutes, then filter with suction, collect the filter cake, wash with deionized water 90 times the mass of gefitinib, and place the filter cake in vacuum drying after suction filtration Dry in an oven at 70°C for 8 hours to obtain gefitinib composite particles.
[0061] Before collecting the gefitinib composite microparticles, inject the good solvent phase into the aqueous phase and stir the suspension for 30 minutes, measure the particle size with a Malvern 2000 particle size analyzer, D90 is 9.846 μm, and carry out X-ray analysis on the dried microp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com