Photocatalyst for complete water splitting and its preparation method and application, reaction method for photocatalytic complete splitting of water and catalytic mixture
A photocatalyst and water splitting technology, applied in the field of photocatalysis, can solve the problems of difficulty in realizing hydrogen production and oxygen production, high recombination rate, etc., and achieve the effect of convenient and accurate configuration of the catalytic system, low cost, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
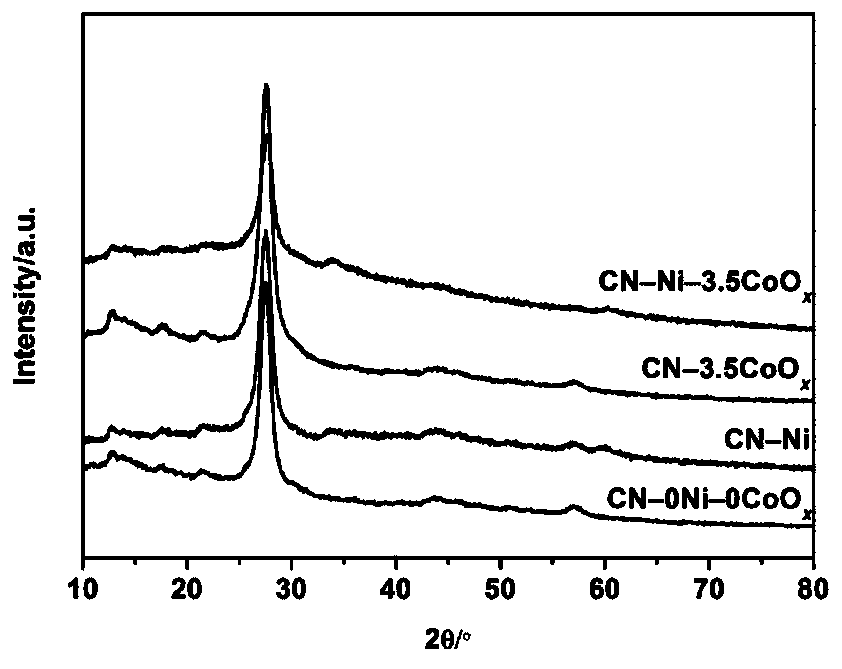
[0038] Step 1: Put 10 g of urea in an alumina crucible, cover it and bake it at 600°C for 3 hours to prepare graphite phase carbon nitride.
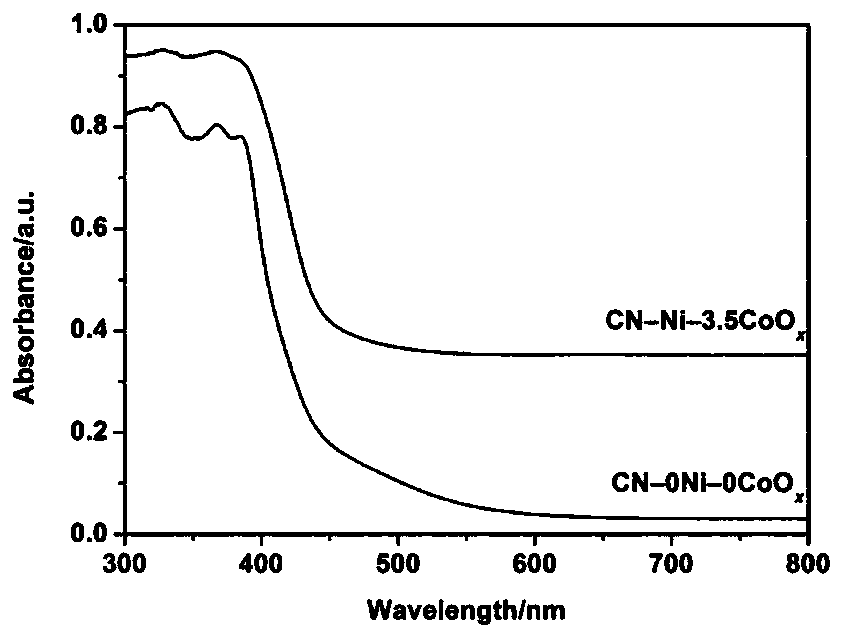
[0039]Step 2: Add the graphite phase carbon nitride powder prepared in step 1 into pure water (CN-0Ni-0CoO x ),carry out testing. Specific steps are as follows:
[0040] 1) Add 50.0 mg of graphitic carbon nitride into a reactor with a volume of 100 mL, and add deionized water so that the total volume of the photocatalytic reaction solution is 80 mL;
[0041] 2) Purge the reactor with argon gas for 30 minutes before lighting to remove the oxygen in the reaction system;
[0042] 3) Turn on the magnetic stirrer and turn on the xenon lamp power supply.
Embodiment example 2
[0044] Step 1: Put 10 g of urea in an alumina crucible, cover it and bake it at 600°C for 3 hours to prepare graphite phase carbon nitride.
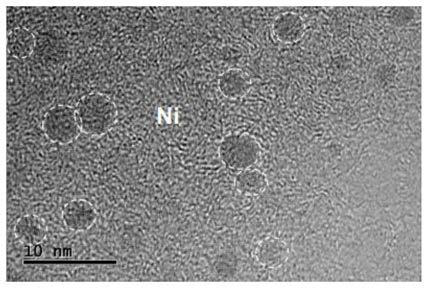
[0045] Step 2: Prepare a metal nickel nanoparticle dispersion. 17.5mg of Ni(NO 3 ) 2 ·6H 2 O. 100.0mg of PVPK30 and 20mL of ethylene glycol were added to a 125mL three-necked flask and completely dissolved, then the three-necked flask was heated and stirred in an oil bath at 120°C, and 63.0mg was added to the solution after the temperature stabilized NaBH 4 (NaBH 4 The mass is 18 times of the mass of metallic nickel), and after heating and stirring for 2 hours, a transparent solution is obtained, which is a dispersion of metallic nickel nanoparticles. Theoretically, the mass of metallic nickel nanoparticles in the dispersion is 3.5 mg.
[0046] Step 3: Add the graphite phase carbon nitride powder prepared in step 1 and the metal nickel nanoparticle dispersion prepared in step 2 into pure water (CN-Ni) for testing. Specific steps ar...
Embodiment example 3
[0051] Step 1: Put 10 g of urea in an alumina crucible, cover it and bake it at 600°C for 3 hours to prepare graphite phase carbon nitride.
[0052] Step 2: Prepare a metal nickel nanoparticle dispersion. 17.5mg of Ni(NO 3 ) 2 ·6H 2 O. 100.0mg of PVPK30 and 20mL of ethylene glycol were added to a 125mL three-necked flask and completely dissolved, then the three-necked flask was heated and stirred in an oil bath at 120°C, and 63.0mg was added to the solution after the temperature stabilized NaBH 4 (NaBH 4 The mass is 18 times of the mass of metallic nickel), and after heating and stirring for 2 hours, a transparent solution is obtained, which is a dispersion of metallic nickel nanoparticles. Theoretically, the mass of metallic nickel nanoparticles in the dispersion is 3.5 mg.
[0053] Step 3: the graphite phase carbon nitride powder prepared in step 1 and the metal nickel nanoparticle dispersion prepared in step 2 and 3 mg of Co(NO 3 ) 2 ·6H 2 O was added to pure water ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com