Polysubstituted benzene compound with biological activity, and preparation method and application thereof
A bioactive, benzene compound technology, applied in the field of multi-substituted benzene compounds and their preparation, can solve the problems of harsh reaction conditions, low specificity, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
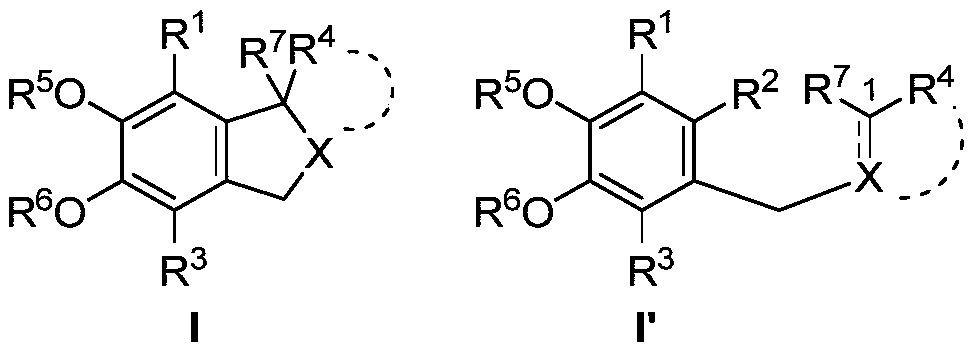
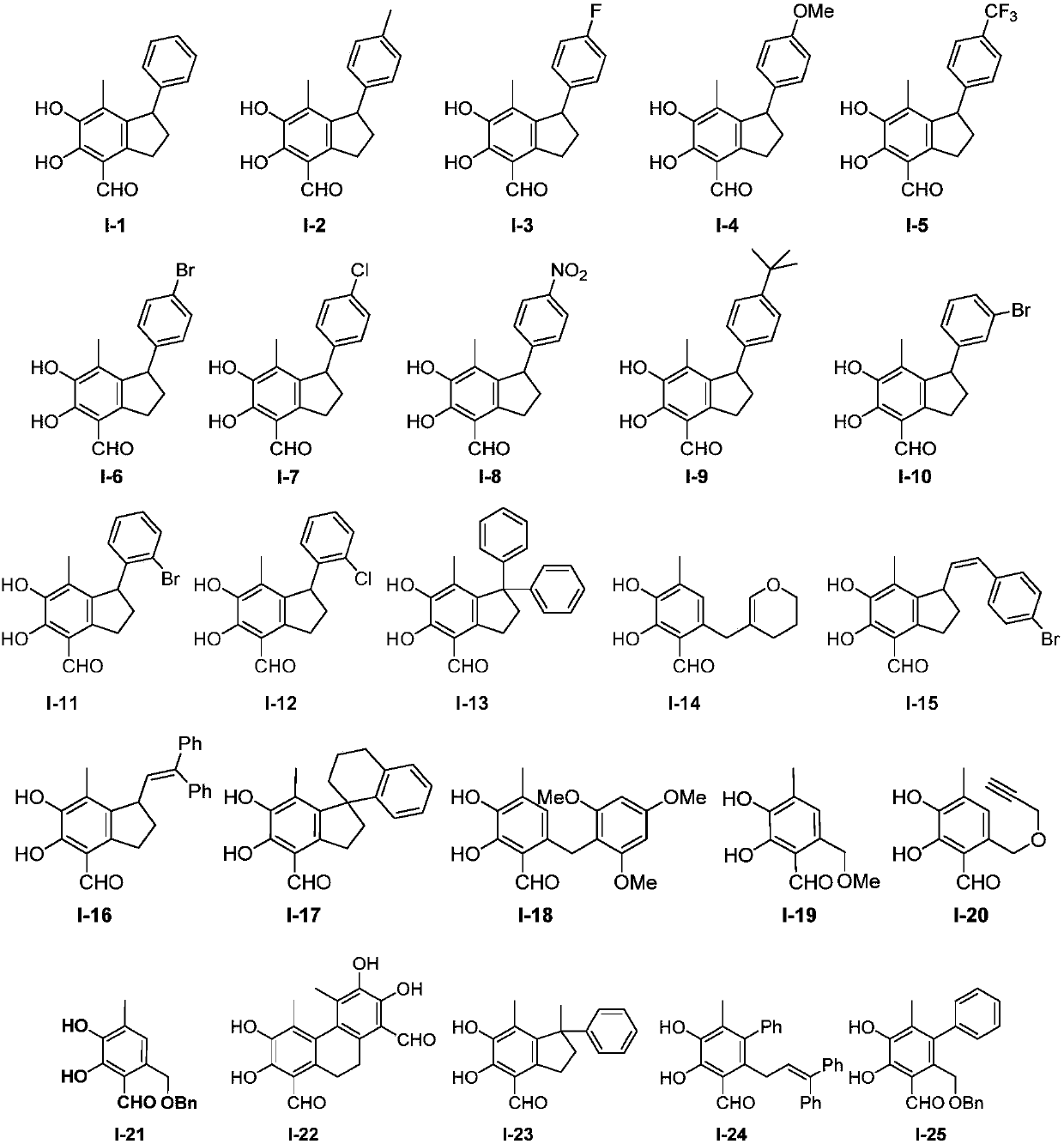
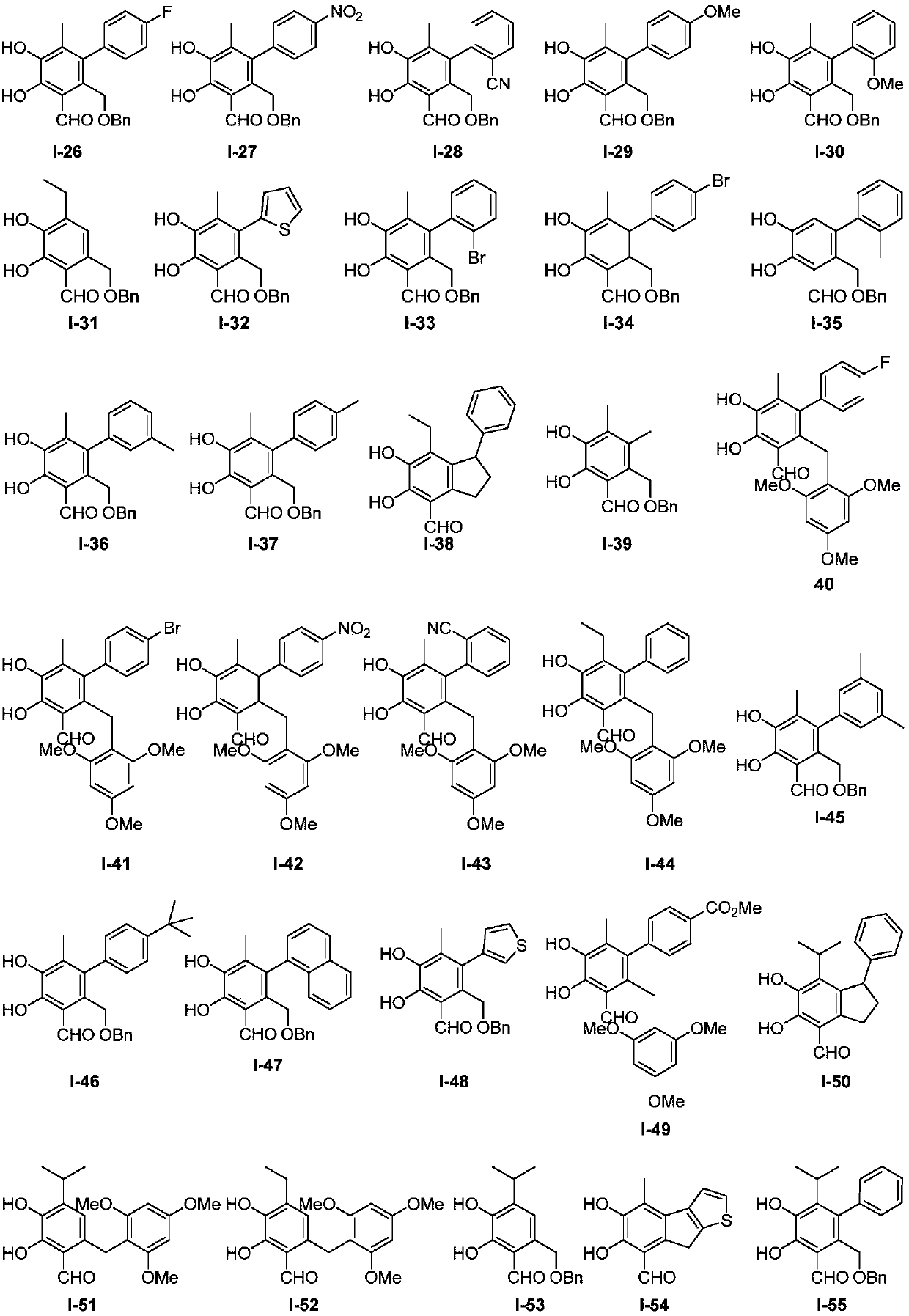
Image
Examples
Embodiment 1
[0108] Preparation of 2-methyl-3-(prop-2-yn-1-yloxy)-4H-pyran-4-one (compound V-1)
[0109] The synthetic route is as follows:
[0110]
[0111] Concrete synthetic steps are as follows:
[0112] 15g of compound VII-1 methyl maltol, 3 equivalents of potassium carbonate and 1.2 equivalents of propargyl bromide were added to 300 milliliters of acetonitrile, heated to 80 ° C for 12 hours; after the reaction, filter with a short silica gel column, reduce The solvent was removed by pressure evaporation, and 17.2 g of compound V-1 was obtained by column chromatography with a yield of 88%.
Embodiment 2
[0114] Preparation of 5,6-dihydroxy-7-methyl-1-phenyl-2,3-dihydro-1H-indene-4-carbaldehyde (compound 1-2)
[0115] The synthetic route is as follows:
[0116]
[0117] Concrete synthetic steps are as follows:
[0118] Add 0.25 mmol of compound V-1, 3.5 equivalents of p-methylstyrene and 1 ml of chlorobenzene into a Shrek tube, heat to 150°C for 13 hours under nitrogen protection, cool down to room temperature, spin dry, and pass through the column to obtain a yellow solid 50.1 mg, namely compound I-2, yield 71%.
Embodiment 3
[0120] Preparation of 1-(3-bromophenyl)-5,6-dihydroxy-7-methyl-2,3-dihydro-1H-indene-4-carbaldehyde (compound I-10)
[0121] The synthetic route is as follows:
[0122]
[0123] Concrete synthetic steps are as follows:
[0124] Add 0.25 mmol of compound V-1, 3.5 equivalents of m-bromostyrene and 1 ml of chlorobenzene into a Shrek tube, heat to 150°C for 13 hours under nitrogen protection, cool down to room temperature, spin dry, and pass through the column to obtain 45 mg of a yellow solid , namely compound I-10, the yield was 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com