Pseudomonas aeruginosa phage and application thereof
A Pseudomonas aeruginosa, phage technology, applied in phage, virus/phage, application and other directions, can solve problems such as untimely treatment and ineffective medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The isolation culture and biological characteristic of embodiment 1 bacteriophage
[0032] (1) Recovery culture of strains and preparation of bacterial suspension
[0033] Pick the frozen bacterial solution of Pseudomonas aeruginosa and streak it in three areas on the NAC plate, separate single colonies, and culture them in a 37°C incubator for 16-24 hours. Pick a single colony, inoculate into 5ml NB broth, and culture in an air shaker at 37°C at 170rpm for 16h to obtain a single bacterial suspension.
[0034] (2) Isolation and purification of phage
[0035] Take feces samples, litter, sewage, fur and other samples and put them into conical flasks filled with NB broth. In an air shaker, shake culture at 170rpm overnight, centrifuge at 10000rpm for 5min, and filter to sterilize with a 0.22μm bacterial filter. Mix the filtrate and the host bacteria, incubate at 37°C for 5 minutes, pour the double-layer plate, put it into a 37°C incubator and incubate it upside down aft...
Embodiment 2
[0054] The mensuration of embodiment 2 phage lysis spectrum and in vitro lysis test
[0055] (1) Determination of phage lysis profile
[0056] The single-spot method was used to determine the lysis profile of the phage, and the steps were as follows: the bacterial suspension of the host bacteria was obtained according to the method in Example 1, and 51 strains of Pseudomonas aeruginosa were respectively derived from mink, fox, cattle, pigs, poultry, rabbits, etc. animal. Add 100 μl of bacterial suspension to the upper layer of agar to prepare a double-layer plate. After the agar solidifies, take 1 μl of phage proliferation solution and drop it on the plate. After natural drying, place it in an incubator at 37°C and incubate it upside down for 6-8 hours. Observation results.
[0057] The results showed that 51 strains of Pseudomonas aeruginosa were used as host bacteria to measure the lysis spectrum, and phage ASP11 could lyse 44 of them, with a lysis rate of 86.27%.
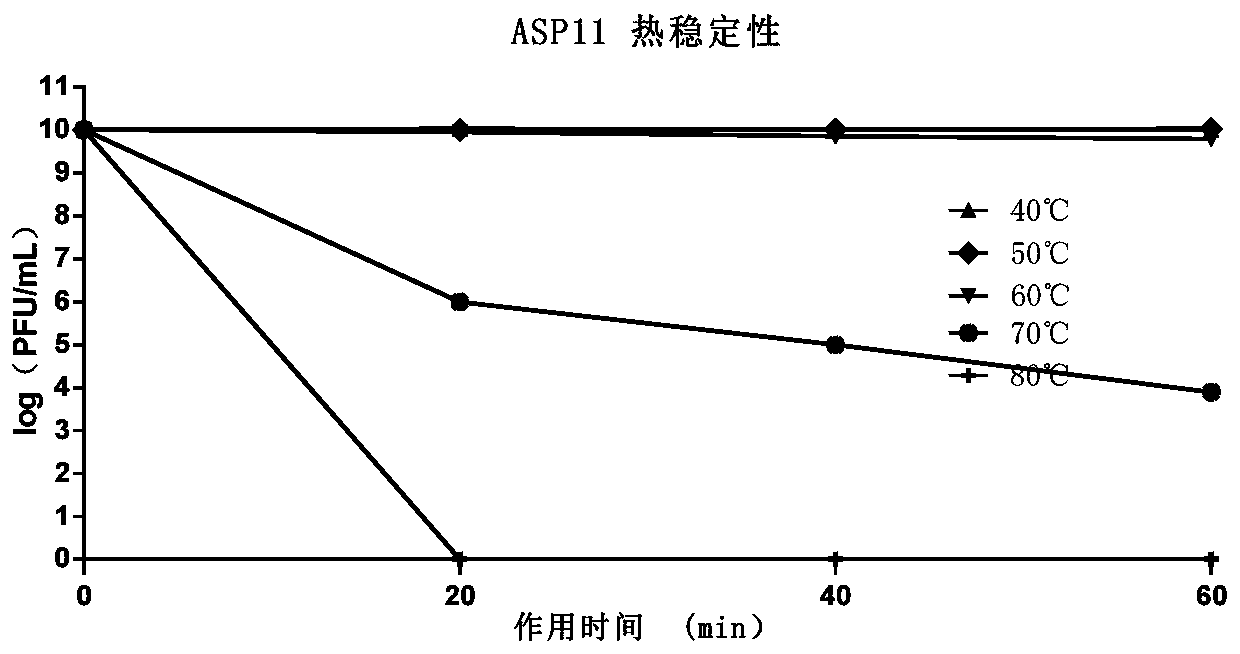
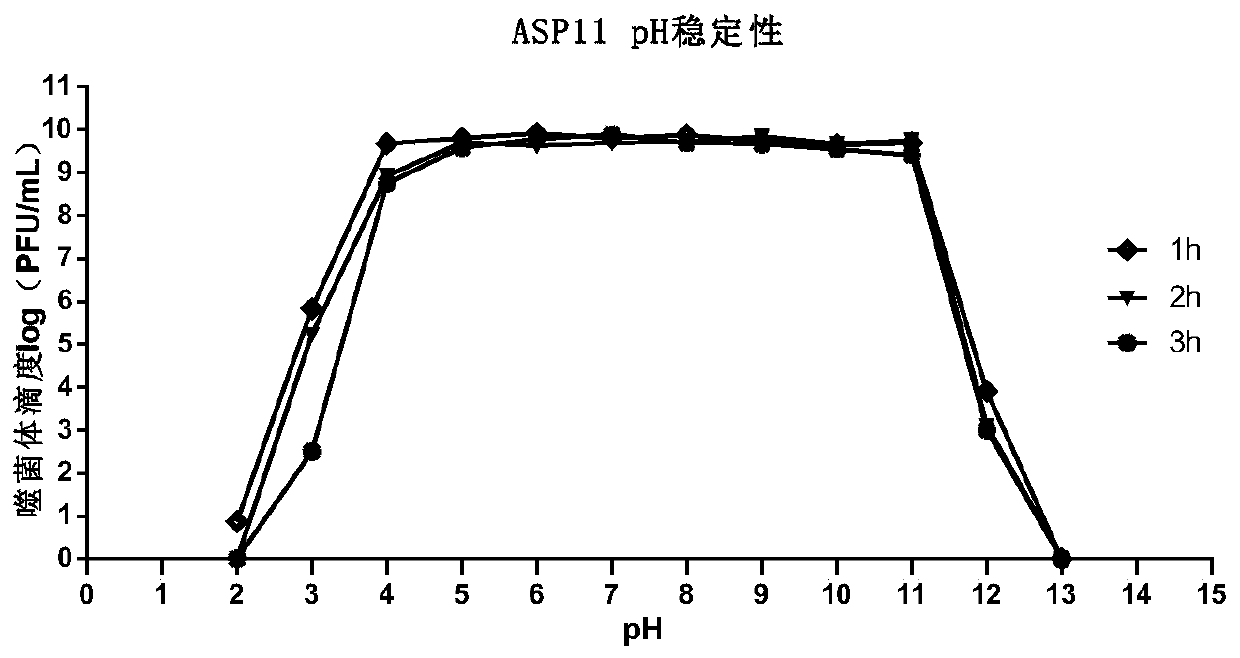
[0058...
Embodiment 3
[0066] The safety test of embodiment 3 bacteriophage
[0067] Select 12 healthy BALB / C mice with a body weight of 18-20 g, 20 males and females, and divide them into experimental group and control group. The mice in each group are divided into male and female mice, respectively, and fed with purified phage proliferation solution (200 μl 10 10 PFU / ml) and normal saline (200 μ l), fed continuously for 7 days, observed the behavioral performance of the mice, and observed the changes of the organs of the mice by autopsy.
[0068] The results showed that the mice in the experimental group and the control group had no abnormal behavior, and the liver, lungs, heart, spleen, kidneys and other organs were normal after autopsy, and there was no significant difference from the control group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com