Application of insect detoxification enzyme protein for degrading organophosphorus pesticide
A technology for detoxifying enzymes and pesticides, applied in the application field of insect glutathione-S transferase protein in the degradation of malathion, can solve the problem of unable to solve the problem of removing malathion pesticide residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Obtaining the locust LmGSTpcF1 gene
[0012] Using the resistant strain locust cDNA as the template, the primers: FF 5'-3': ATGCCAGTCACCCTC, and FR 5'-3': CTACGGAGCCAGATG were cloned to obtain the full-length gene of LmGSTpcF1. The system is: 2 × Taq PCR MasterMix (25 μL), DNA template (1 μL), primers (1 μL each), ddH 2 O (21 μL) for a total of 50 μL. After recovery, purification and sequencing of the product, SEQ ID NO: 1 was obtained.
Embodiment 2
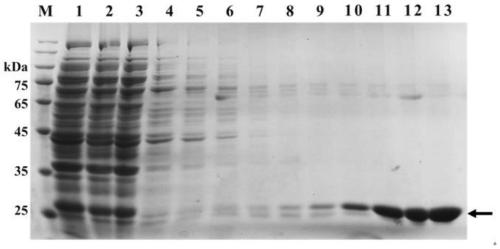
[0013] Example 2: Expression and purification of LmGSTpcF1
[0014] According to SEQ ID NO: 1, primers containing BamHI and HindIII restriction sites were designed, WF5'-3': TAGGATCCATGCCAGTCACCCTC and WR5'-3': ATAAGCTTCTACGGAGCCAGATG for recombinant plasmid construction. After PCR, the product was digested with BamHI and HindIII, and recovered by 1.5% agarose gel electrophoresis. The prokaryotic expression vector pET28a was also processed simultaneously. After mixing the recovered products, buffer and T4 ligase were added, ligated overnight at 4°C, and transformed into E. coli DH5α competent cells. The strains with accurate verification results were selected, expanded and cultured, the recombinant plasmids were extracted, and transformed into E. coli BL21(DE3) competent cells. Pick positive colonies, and expand the culture after PCR verification. The positive monoclonal colonies were inoculated into liquid LB medium (containing 50 μg·mL -1 kanamycin) in a test tube, cultu...
Embodiment 3
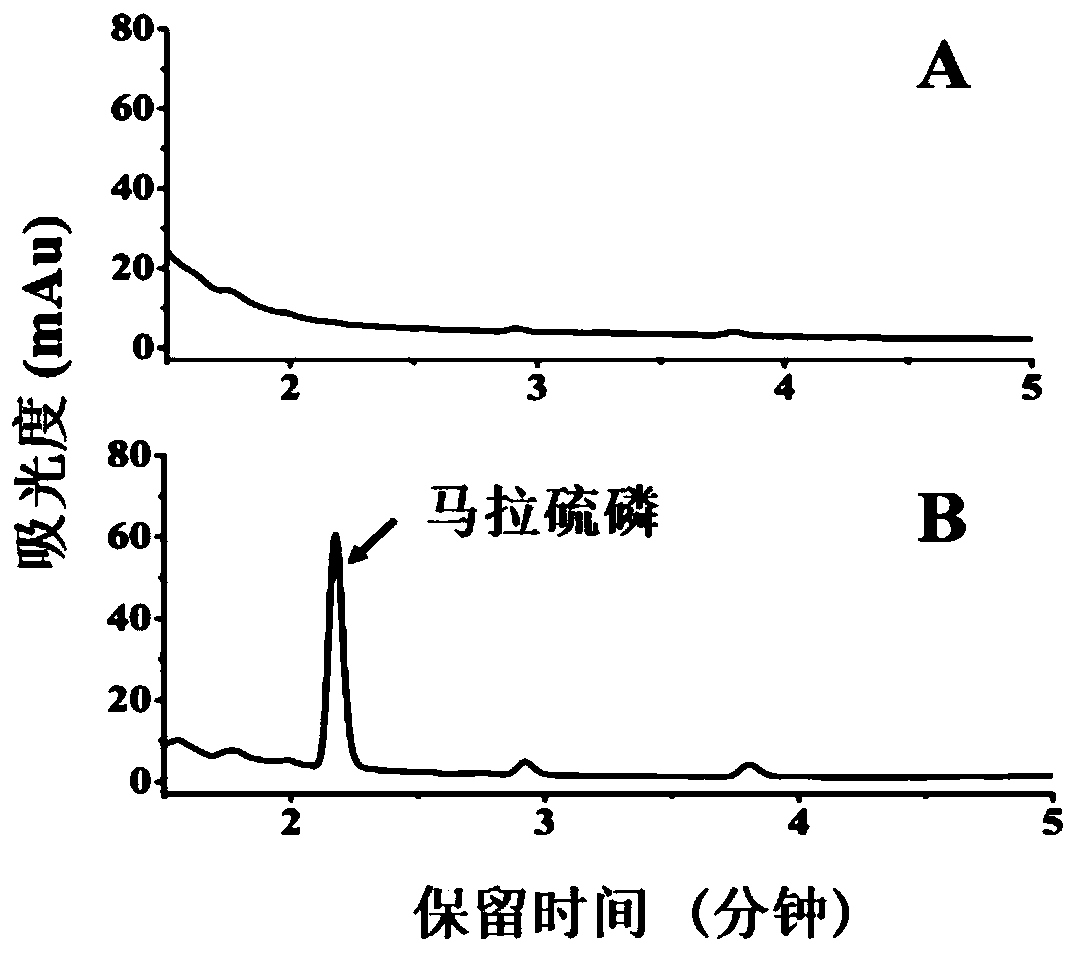
[0016] Example 3 Metabolism detection of malathion by LmGSTpcF1
[0017] The extent of malathion metabolism by LmGSTpcF1 was calculated using the peak area of the pesticide detected by HPLC. The chromatographic detection conditions were that mobile phase A was acetonitrile, and B was 0.1% formic acid water. The elution gradient was A:B=80:20 isocratic elution for 8min; detection wavelength 210nm; column temperature 35℃; sample volume 2uL; flow rate 0.2mL / min.
[0018] The samples were divided into two groups, A and B. Group A was a normal reaction group (LmGSTpcF1+GSH+malathion); group B was a protein-free control group (PBS+GSH+malathion) with PBS buffer instead of protein. The 200uL reaction solution includes: 20uL 1.6mg / mL mGSTpcF protein (32ug); 15uL 20mg / mL malathion (300ug), 10uL 50mM GSH (2.5mM), PBS to make up the volume to 200uL. After the reaction solution was mixed, it was placed at 27°C, 1 200 r / min, and incubated for 3 h; after the reaction, 400 μL of acetoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com