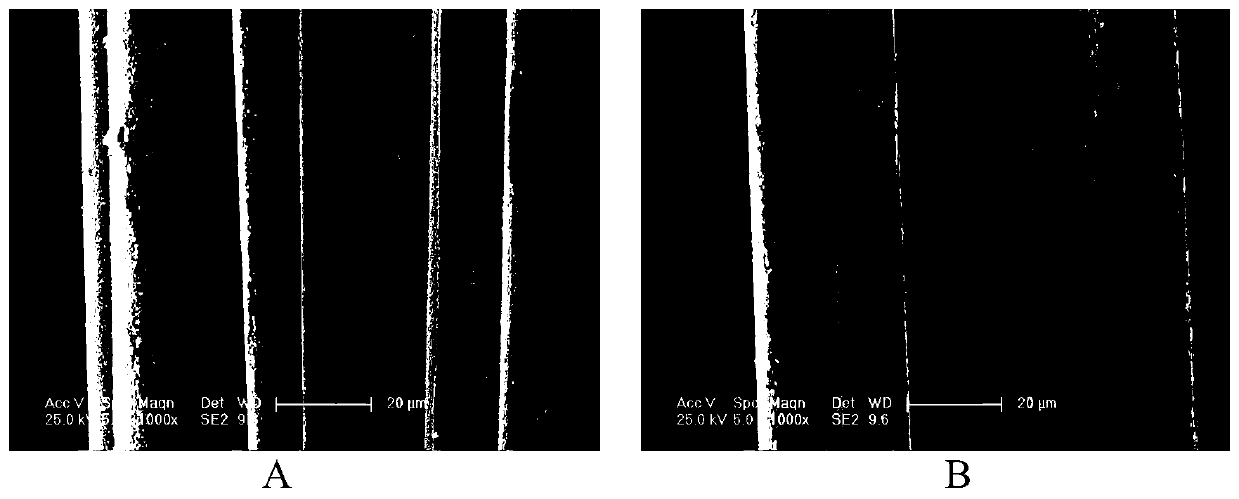
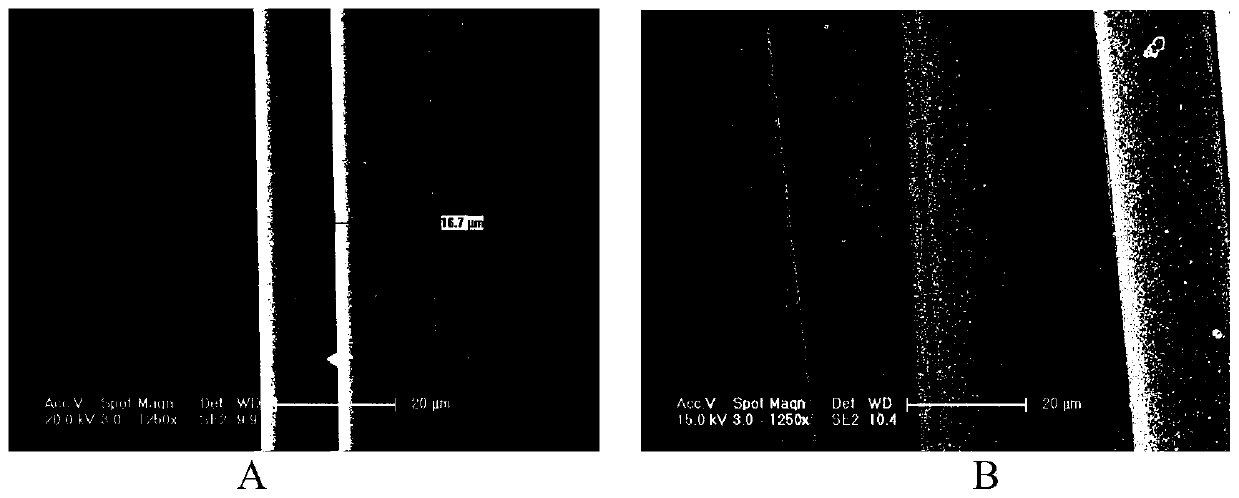
Conductive gold-coated polyimide fiber and preparation method thereof
A polyimide fiber and coating technology, which is applied in the field of conductive gold-coated polyimide fiber and its preparation, can solve the problems of no polyimide fiber gold plating, limited use, etc. The effect of maintaining mechanical properties, wide operating temperature range, and obvious attenuation of mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention provides a method for preparing conductive gold-coated polyimide fibers described in the above technical solution, comprising the following steps:
[0045] Step A: Alkaline treatment and cleaning of polyimide fibers to obtain polyimide fibers with carboxylate on the surface;
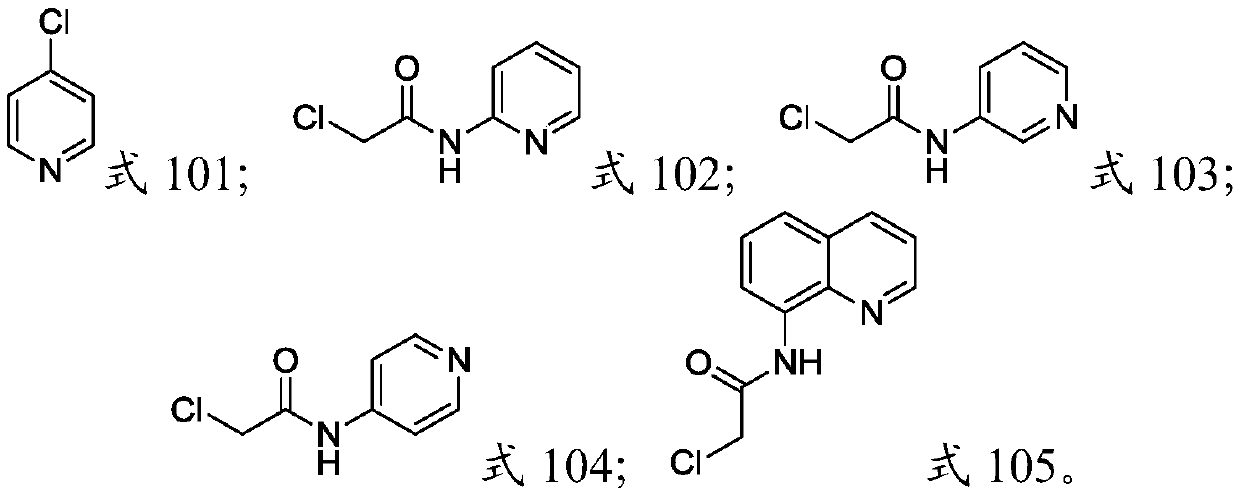
[0046] Step B: placing the fibers obtained in step A in a solution of pyridine derivatives or quinoline derivatives to react, and washing to obtain ester-bonded polyimide fibers containing pyridine derivatives or quinoline derivatives on the surface;
[0047] Step C: placing the fibers obtained in step A or step B in a palladium salt solution for palladium ion exchange or complexation, and cleaning to obtain polyimide fibers containing palladium ions on the surface;
[0048] Step D: reducing and cleaning the polyimide fiber containing palladium ions on the surface to obtain a polyimide fiber with a palladium metal particle layer on the surface;
[0049] Step E: chemically go...
Embodiment 1
[0059] Dissolve 2-aminopyridine (47.0g, 0.5mol) and triethylamine (104.2mL, 0.75mol) in dichloromethane (300.0mL), control the reaction temperature at 0℃~20℃ and add 2-chloroacetyl chloride dropwise (62.1g, 0.55mol), react at room temperature for 3h after dropping. Add water (100.0 mL) and continue stirring for 1 h, separate the water phase, wash the organic phase with saturated aqueous sodium bicarbonate (100.0 mL), dry the organic phase with anhydrous sodium sulfate, filter off the desiccant, distill off dichloromethane, and dry in vacuo A reddish solid (76.8 g, yield: 90%), the pyridine derivative represented by the structure of Formula 102, was obtained. 1 H NMR (400MHz, CDCl 3 ):δ4.20(s,2H),7.10-7.09(m,1H),7.77-7.72(m,1H),8.20(d,1H,J=4.4Hz),8.32-8.31(m,2H), 8.93(s,1H).
Embodiment 2
[0061] Using 3-aminopyridine as the reaction raw material, the preparation process is the same as that in Preliminary Example 1. The product is a white solid, that is, the pyridine derivative shown in the structure of formula 103. The yield: 80.2 g, yield: 94%. 1 H NMR (400MHz,D 2 O): δ9.16(d, J=3Hz, 1H), 8.42(d, 1H, J=6Hz), 8.40(d, 1H, J=9Hz), 7.90(dd, 1H, J=9Hz, 6Hz) ,4.25(s,2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com