Fangchinoline-carbamate derivative with bactericidal activity
A technology of carbamates and fangchinoline base, which is applied in the field of synthesis of fangchinoline-carbamate derivatives, achieving the effect of high yield and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
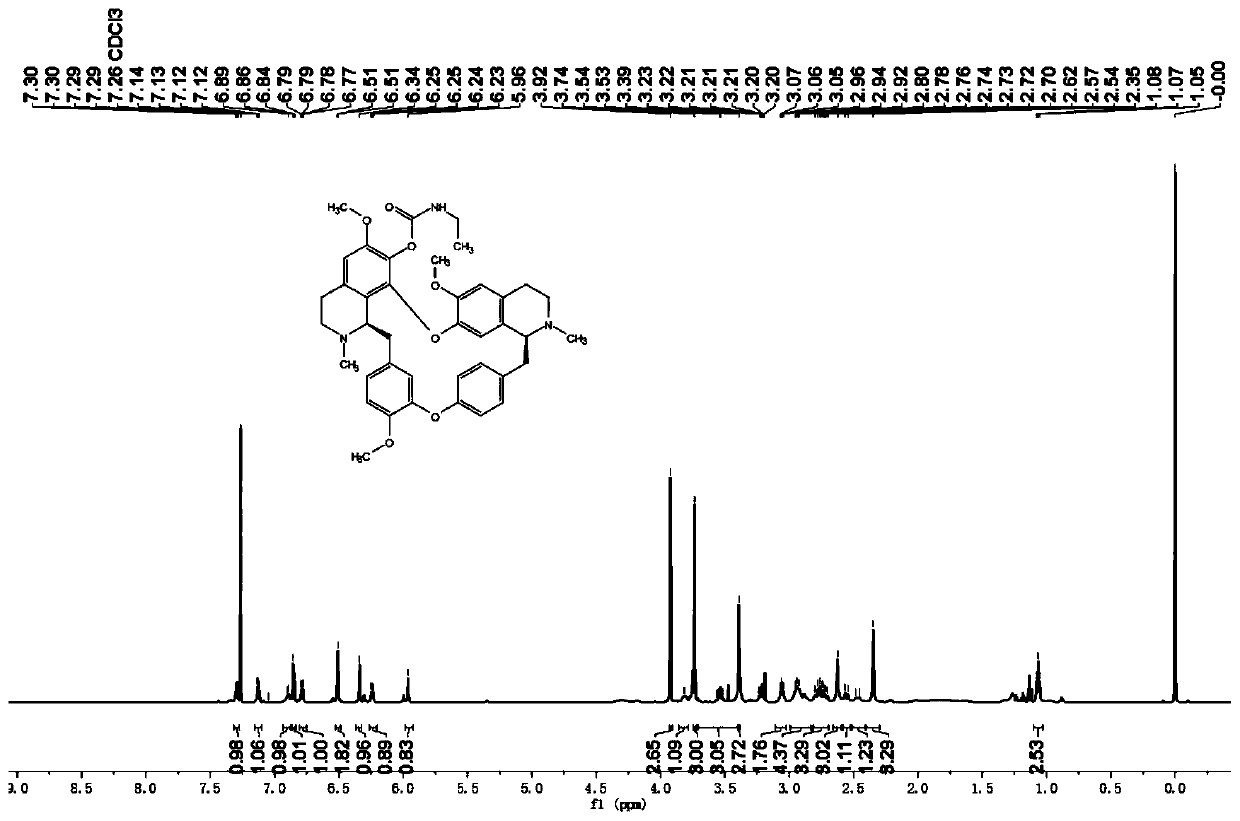
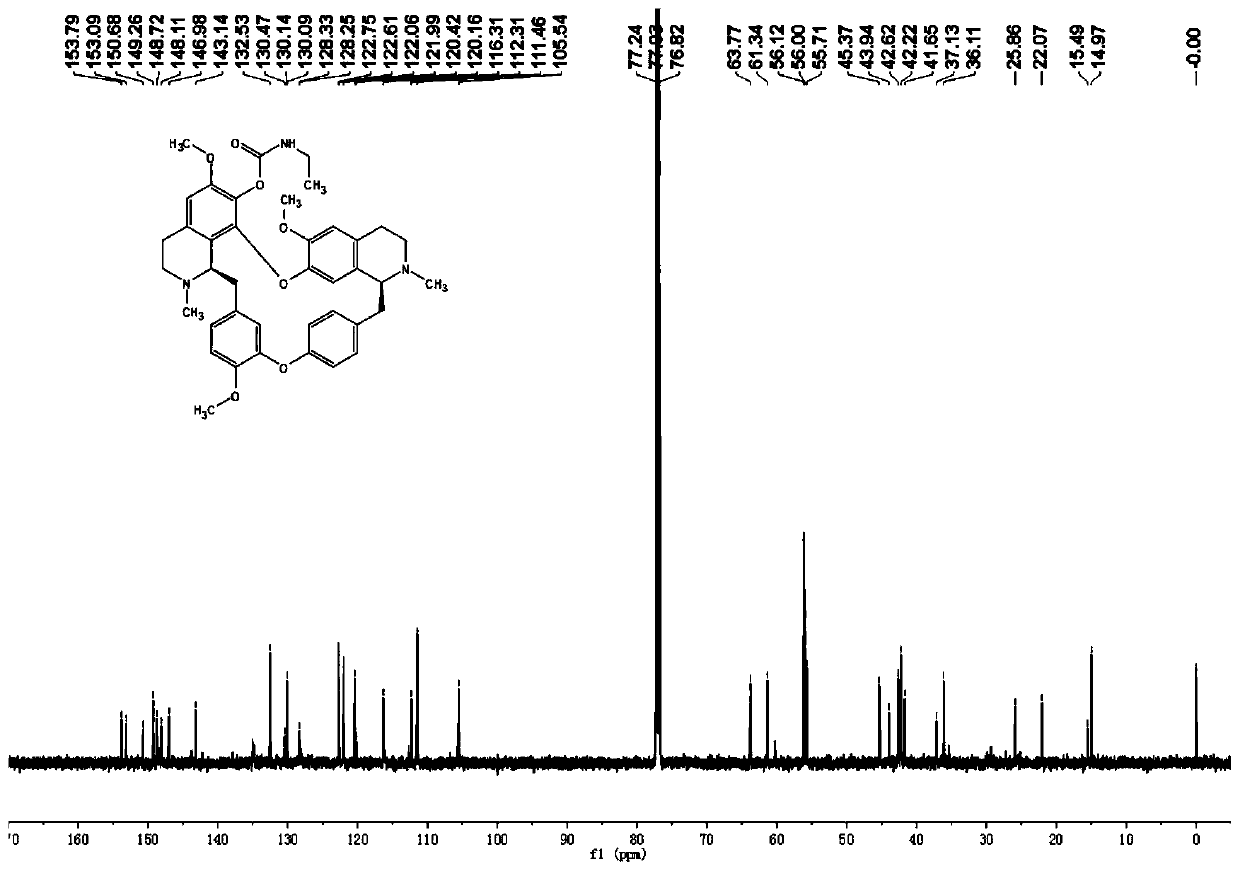
[0045] The preparation of embodiment 1 compound I-a
[0046] The compound I-a prepared in this embodiment has the structure shown in the following formula:
[0047]
[0048] Synthetic method: Dissolve tetrandrine (2.53g, 4.16mmol) in dichloromethane (CH 2 Cl 2 ,15mL), then add triethylamine (NEt 3 , 1.26g, 12.47mmol) and ethyl isocyanate (12.47mmol) and the mixture was warmed to room temperature, under N 2 Stir under atmosphere for 24 hours. The reaction mixture was quenched with ice water and washed with dichloromethane (CH 2 Cl 2 , 3×20mL) extraction. The combined organic phases were washed with brine (2×30 mL), and washed over anhydrous sodium sulfate (Na 2 SO 4 ) to dry overnight. After removing the solvent in vacuo, the crude product was chromatographed on silica gel (petroleum ether:ethyl acetate=10:1, v / v) to obtain the target compound I-a.
[0049] The instrument used for infrared spectrum (IR) measurement is the AVATAR 330 type infrared spectrometer (KBr ...
Embodiment 2
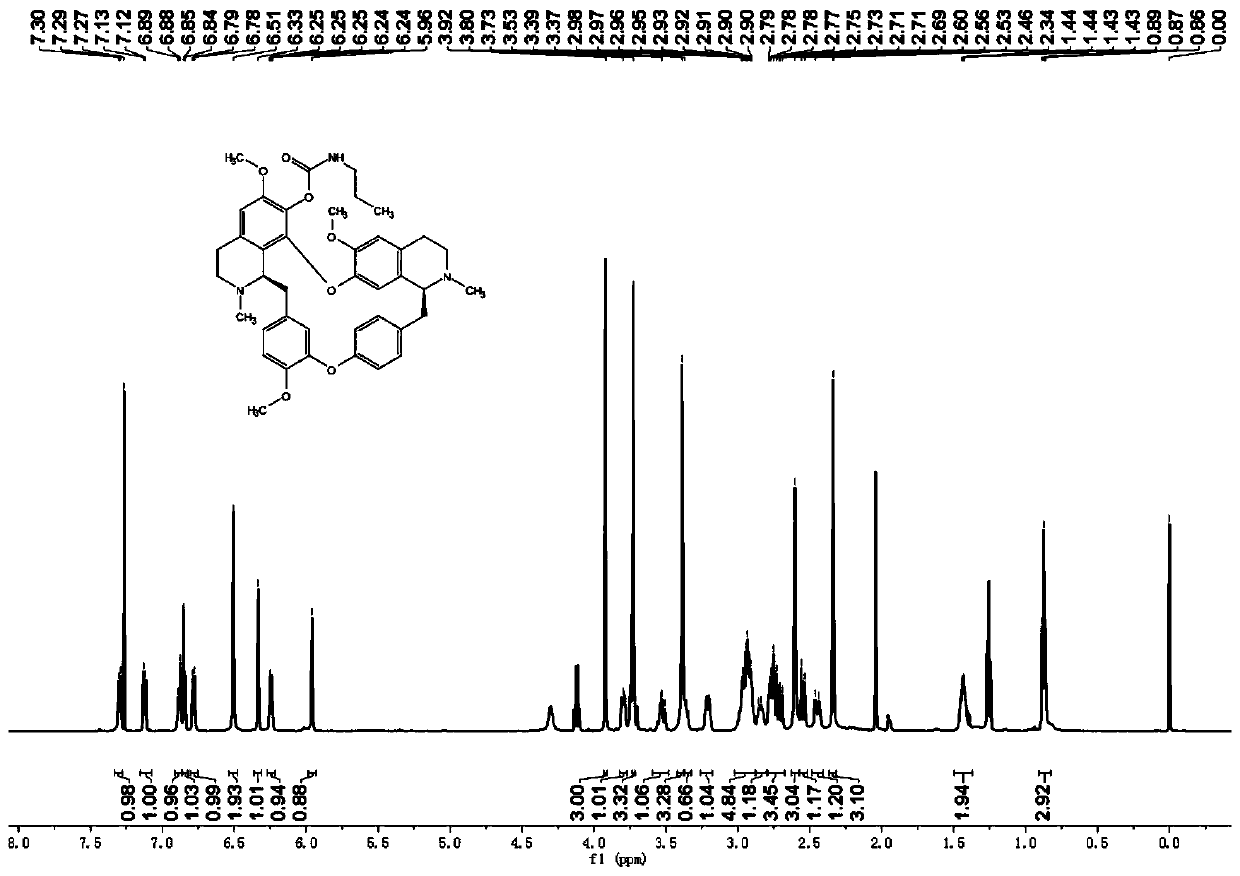
[0051] The preparation of embodiment 2 compound I-b~I-1
[0052] The synthetic method of compound I-b~I-l is the same as I-a, wherein ethyl isocyanate is changed into corresponding R 1 group, R 1 They are: propyl, isopropyl, tert-butyl, phenyl, o-methylphenyl, m-methylphenyl, p-methylphenyl, p-methoxyphenyl, p-chlorophenyl, p-trifluoro Methylphenyl, p-trifluoromethoxyphenyl. The physical and chemical properties of the synthesized compounds are shown in Table 1 below.
[0053] The physicochemical property of table 1 compound I-a~I-l
[0054]
[0055] I-a: yellow powdery solid; yield 89%; melting point::154-155°C; IR: 1739 (C=O); HRMS (ESI), m / z: 680.3386[M+H] + (calcd for C 40 h 46 N 3 o 7 :680.3330); 1 H NMR (600MHz, CDCl 3 )δ: 7.29 (1H, dd, J = 8.2, 2.2Hz), 7.13 (1H, dd, J = 8.2, 2.6Hz), 6.90 (1H, d, J = 7.4Hz), 6.85 (1H, d, J =8.2Hz),6.79(1H,dd,J=8.2,2.6Hz),6.51(2H,s),6.34(1H,s),6.24(1H,dd,J=8.2,2.2Hz),5.96(1H ,s),3.92(3H,s),3.81(1H,d,J=7.8Hz),3.74(3H,s),3.72-...
Embodiment 3
[0067] Embodiment 3 preliminary screening test of antibacterial activity
[0068] Tested strains: Gibberella zeae, Phoma adianticola, Phomopsis adianticola, Altermaria alternata, Colletotrichum fructicola, tea tree Pestalotiopsis theae.
[0069] Production medium: Wash and peel the potatoes, cut into pieces, weigh 400g in a container, add water to boil, and keep boiling slightly for 15-20min, stir moderately, and stop heating. Filter in a beaker with three layers of gauze, add 40g of glucose and 40g of agar, stir to dissolve, transfer to a 2000mL volumetric flask and set the volume to 2000mL. Add 198mL of filtrate to ten 250mL Erlenmeyer flasks, wrap them with sterile parafilm, seal them again with newspaper, exhaust the air with a vertical pressure steam sterilizer at 101°C, and sterilize at 121°C for 30 minutes for later use. All required Petri dishes and pipette tips were sterilized under the same conditions and dried for later use.
[0070] Dispensing and inoculation: W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com