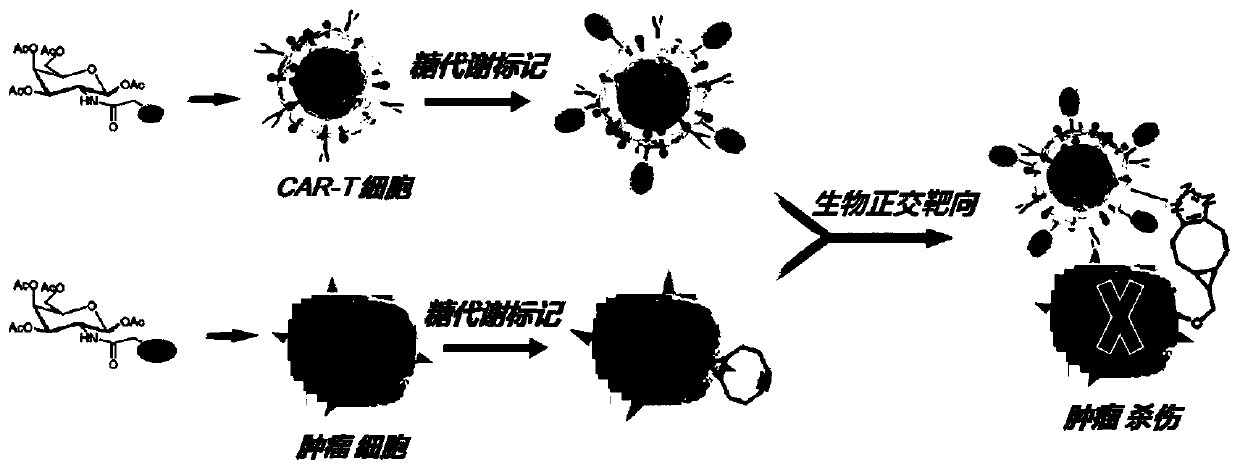
Manually target-modified CAR-T cells and preparation method and application thereof
A technology of cells and modifiers, applied in the field of artificially modified CAR-T cells and its preparation, can solve the toxic and side effects of the body, can not solve the problems of tumor off-target effects, cell activity reduction, etc., to achieve the protection of functional activity, Overcoming off-target phenomena and tumor immune escape, avoiding effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of sugar / lipid derivatives modified by chemical reporter groups
[0049] Synthesis of carbohydrate derivatives: We first synthesized 4-nitrophenyl chloroformate-activated bicyclic [6.1.0 ] Nonene (nPC-BCN). The nPC-BCN was then coupled to a glycan (mannose) to give N-BCN-carbonyl-D-mannosamine (ManN-BCN). To increase cellular uptake, these unnatural glycans were peracetylated to form tetra-O-acetyl compounds and lyophilized to obtain tetraacetylated-N-BCN-carbonyl-D-mannosamine (Ac 4 ManNBCN) powder.
[0050] Synthesis of lipid derivatives: mainly refer to the method disclosed in the literature Analytical Chemistry 2013 85 (10), 5263-5270. The specific preparation method is as follows: dissolve a certain amount of 1,2-dibromoethane and sodium azide in DMF, react at 80°C for 20 hours, add ice water and sodium chloride solution after the reaction, and extract to obtain the intermediate 2' - bromoazidoethane. Dissolve the obtained 2'-bromoazid...
Embodiment 2
[0051] Example 2: Artificial bioorthogonal targeted CAR-T cells for the treatment of hematological tumors
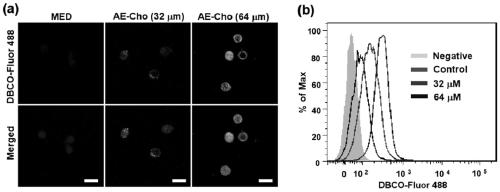
[0052] Azide group modification of CAR-T cells: CAR-T cells were co-incubated with azide-modified choline derivatives (AE-Cho) at a final concentration of 32-64 μM at 37°C for 48 h, and the lipids of T cells Metabolic process, the azide group is embedded in the surface of the CAR-T cell membrane (N 3 -CAR-T). The excess modifier was washed away with PBS buffer, and the cells were stained with DBCO-Fluor 488. Cells were harvested and analyzed by confocal and flow cytometry for the expression of azide molecules on the cell surface. Such as image 3 As shown, azide molecules are highly expressed on the surface of CAR-T cells.
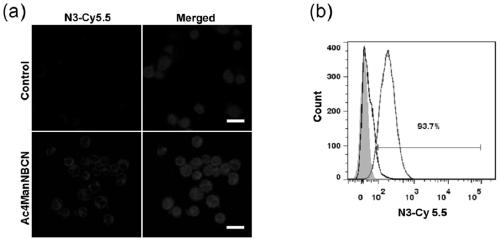
[0053] Glucose metabolism modification of hematoma cells (Raji): Luci-Raji cells carrying luciferase were mixed with BCN group-modified mannose (Ac 4 ManNBCN) were co-incubated at 37°C for 48 hours, and the -BCN group was embedded on the tumor...
Embodiment 3
[0056] Example 3: Artificial bioorthogonal targeted CAR-T cells for the treatment of solid tumors
[0057] Azide group modification of CAR-T cells: CAR-T cells were co-incubated with azide-modified choline derivatives (AE-Cho) at a final concentration of 64 μM at 37°C for 48 h, through the lipid metabolism of T cells process, the azide group is embedded in the surface of the CAR-T cell membrane (N 3 -CAR-T). The excess modifier was washed away with PBS buffer, and the cells were stained with DBCO-Fluor 488. Cells were harvested and analyzed by confocal imaging and flow cytometry for the expression of azide molecules on the cell surface. Such as image 3 As shown, azide molecules are highly expressed on the surface of CAR-T cells.
[0058] Glycometabolic Modification of Raji Solid Tumors: Divide 1 × 10 7 Luci-Raji cells carrying luciferase subcutaneously in tumor-bearing NOD / SCID mice. When the tumor volume reaches 100mm 3 Around time, by intratumoral injection of 5-10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com