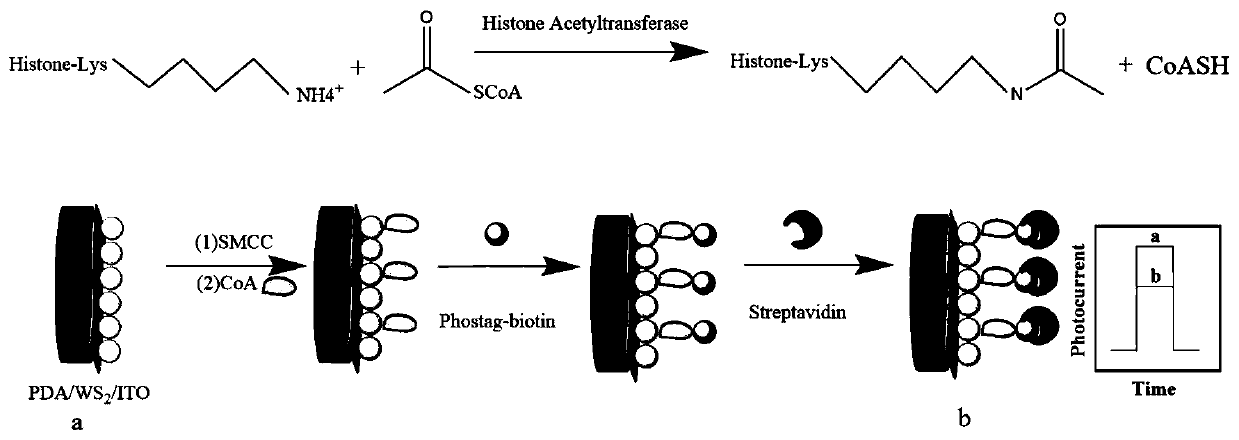
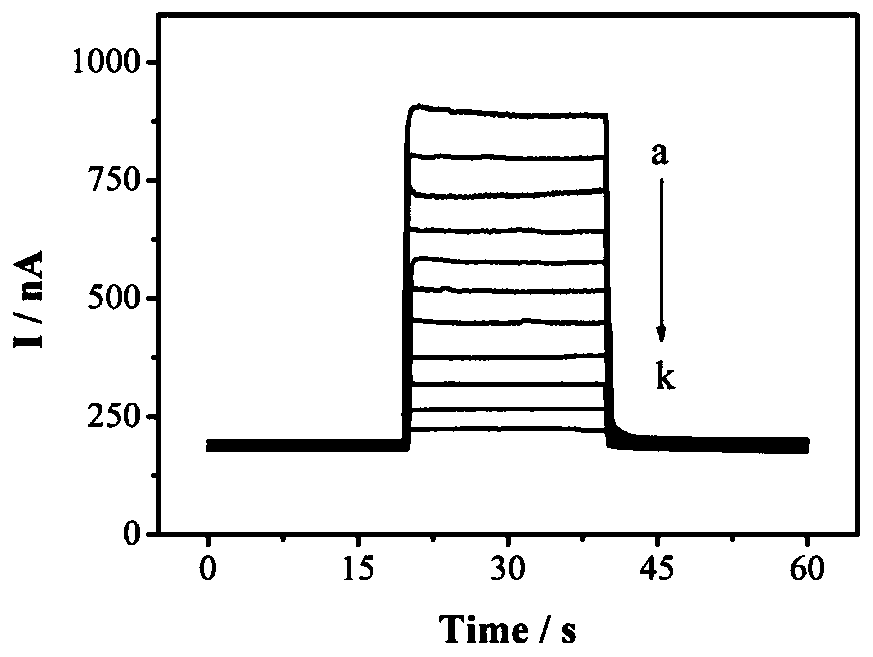
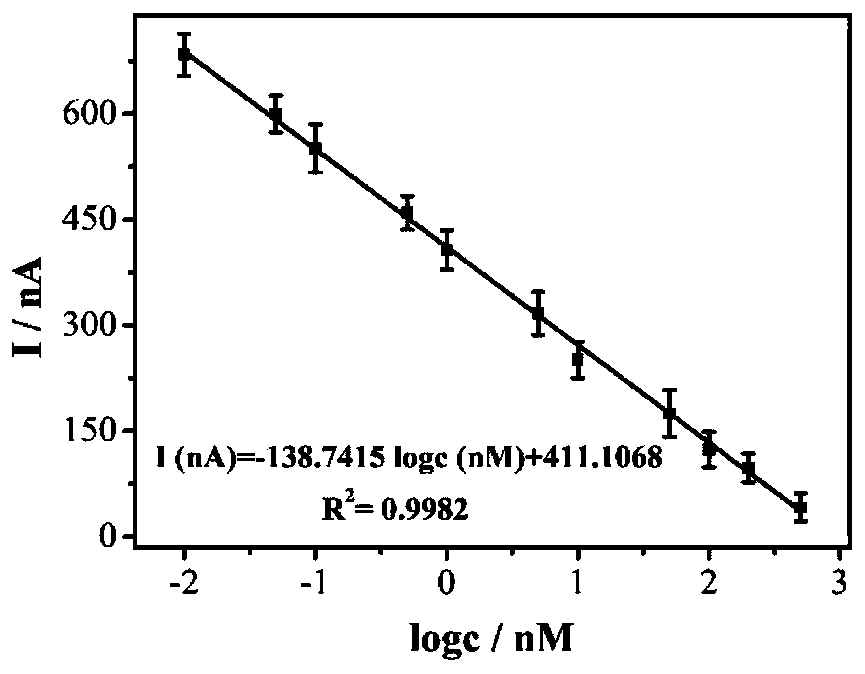
Photoelectrochemical biosensor for detecting histone acetyltransferase activity and preparation method thereof
A technology of acetyltransferase and biosensor, applied in the direction of material electrochemical variables, scientific instruments, instruments, etc., can solve the problems of health hazards for operators, expensive antibody reagents, cumbersome sample processing, etc., to improve detection sensitivity and realize small instruments The effect of simple chemicalization and detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] (1) Preparation of catalytic reaction solution: mix 20-50 μL of different concentrations of histone acetyltransferase, 20-50 μL of substrate peptide (concentration of 10-40 μM), 10-40 μL of acetyl-CoA (concentration of 100-300 μM) well mixed. The mixture is then incubated at 20-50°C for 1-5 hours. The resulting solution was labeled as catalytic reaction solution.
[0053] (2) Preparation of exfoliated tungsten sulfide: weigh 50-300mg WS 2 And 50-300mg of polyacrylic acid dissolved in 20-60mL of water, ultrasonic 2-6h. Centrifuge the green dispersion at 2000-10000rpm for 5-30min, collect the supernatant and centrifuge at 5000-10000rpm for 5-30min, wash the obtained precipitate with deionized water several times to remove the residue, and finally dry it under vacuum.
[0054] (3) Preparation of polydopamine: Dissolve 100-200mg of dopamine hydrochloride in 50-100mL of deionized water, under vigorous stirring, take 500-1000μL of 1-3mol / L NaOH solution and add it to the a...
Embodiment 1
[0072] Example 1: Histone acetyltransferases catalyze acetylation of substrate peptides
[0073] Mix 20 μL of 50 nM histone acetyltransferase, 20 μL of substrate peptide (concentration of 20 μM), and 20 μL of acetyl-CoA (concentration of 100 μM). The mixture was then incubated at 37°C for 1 hour. The resulting solution was labeled as catalytic reaction solution.
Embodiment 2
[0074] Embodiment 2: Preparation of exfoliated tungsten sulfide
[0075] Weigh 50mg WS 2 And 300mg of polyacrylic acid dissolved in 60mL of water, ultrasonic 6h. The green dispersion was centrifuged at 3000rpm for 10min, the supernatant was collected and centrifuged at 5000rpm for 15min, the obtained precipitate was washed several times with deionized water to remove the residue, and finally dried under vacuum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com