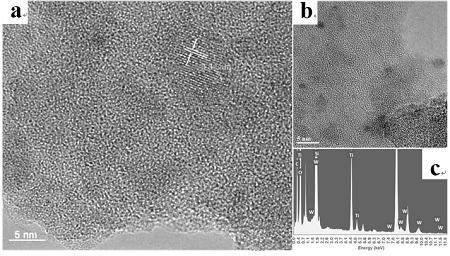
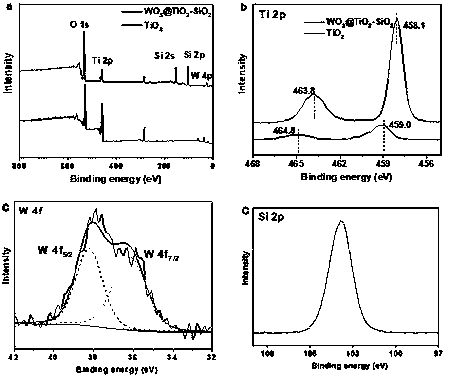
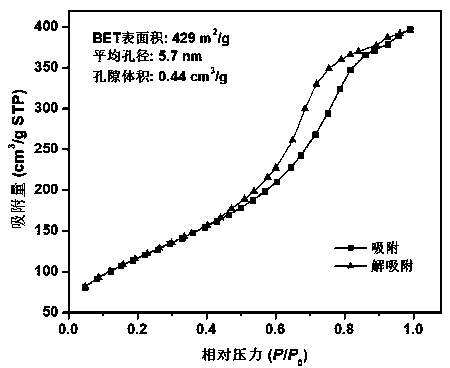
Preparation of tungsten/titanium oxide photocatalytic material based on SiO2 with large specific surface area
A technology of photocatalytic materials and specific surface area, applied in the field of photocatalysis, can solve the problems of slow natural photodegradation of pollutants, and achieve the effects of reducing internal recombination rate, improving utilization rate and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 (comparative example): Blank TiO 2 preparation of
[0026] After mixing tetrabutyl titanate and anhydrous methanol for 30 min, the mixed solution of nitric acid and pure water (volume ratio 0.13:1) was added dropwise to the mixed solution, and the stirring was continued for 2 hours. The reacted mixed solution was placed under the condition of stirring and heating to evaporate methanol, and the obtained dry solid was washed several times, then heat-treated at 800 °C for 2 hours, and ground for the experiment of photocatalytic degradation of phenanthrene.
Embodiment 2
[0027] Example 2: TiO 2 -SiO 2 preparation of
[0028] A certain amount of SiO 2 After the aerosol, tetrabutyl titanate and anhydrous methanol were mixed for 30 min, the mixed solution of nitric acid and pure water (volume ratio 0.13:1) was added dropwise to the mixed solution, and the stirring was continued for 2 hours. The reacted mixed solution was placed under the condition of stirring and heating to evaporate methanol, and the obtained dry solid was washed several times, then heat-treated at 800 °C for 2 hours, and ground for the experiment of photocatalytic degradation of phenanthrene.
Embodiment 3
[0029] Example 3: WO 3 @TiO 2 -SiO 2 preparation of
[0030] A certain amount of SiO 2 After the aerosol, tetrabutyl titanate, tungsten chloride and anhydrous methanol were mixed for 30 min, the mixed solution of nitric acid and pure water (volume ratio 0.13:1) was added dropwise to the mixed solution, and the stirring was continued for 2 hours. The reacted mixed solution was placed under the condition of stirring and heating to evaporate methanol, and the obtained dry solid was washed several times, then heat-treated at 800 °C for 2 hours, and ground for the experiment of photocatalytic degradation of phenanthrene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com