An ionic liquid-assisted preparation of pt/tio 2 New methods and uses of catalysts
A technology of ionic liquids and catalysts, applied in the field of materials, can solve problems such as complex steps, and achieve the effects of rich oxygen vacancies, strong light absorption ability, and small crystal size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The Pt / TiO of this embodiment 2 The synthesis method is as follows:
[0041] Synthesis of Pt / TiO 2 The ionic liquid used is 1-ethyl-3-methylimidazolium tetrafluoroborate (EmimBF 4 ), as shown in formula (II).
[0042]
[0043] 6.25mg chloroplatinic acid hexahydrate (H 2 PtCl 6 ·6H 2 O) Dissolve in a mixture of 1.25g 1-ethyl-3-methylimidazolium tetrafluoroborate and 1.25g deionized water, add 1mL tetrabutyl titanate dropwise while vigorously stirring, and transfer the above mixture into In a 15mL autoclave, the reaction was stirred at 150°C for 12h. After the reaction, the reaction kettle was naturally cooled to room temperature, and the reaction suspension was centrifuged to obtain a solid, which was fully washed with deionized water for 5 times, and vacuum-dried overnight at 80°C to obtain the final product.
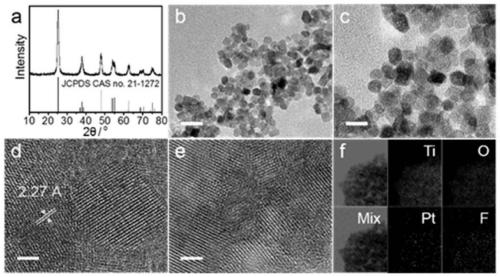
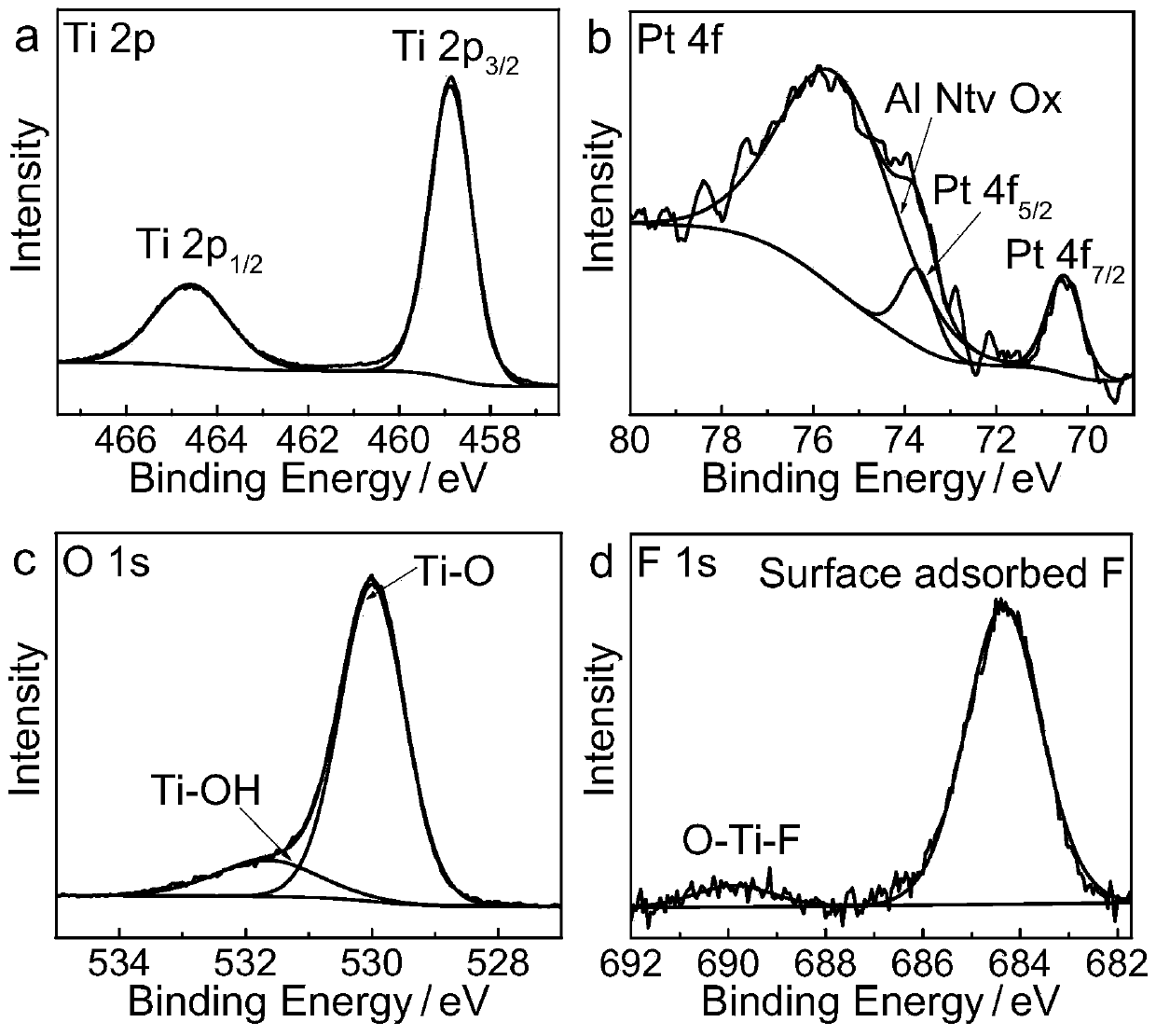
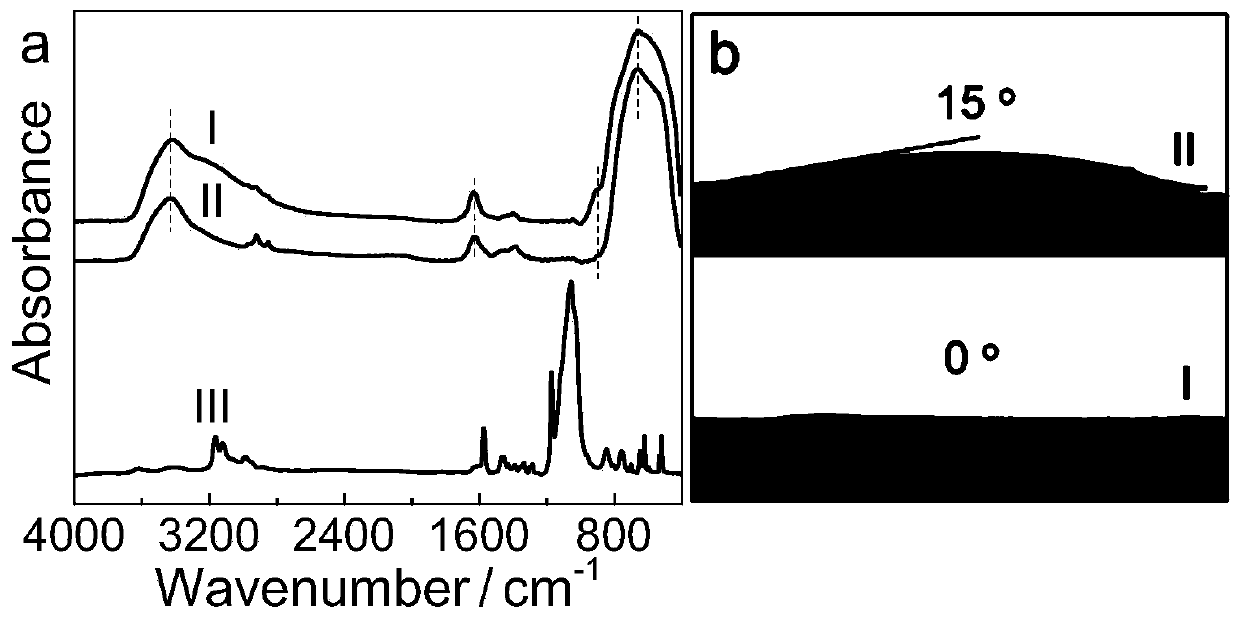
[0044] The obtained product was characterized by X-ray diffraction, transmission electron microscope, high-resolution transmission electron microscope ...
Embodiment 2
[0049] The Pt / TiO of this embodiment 2 The synthesis method is as follows:
[0050] Synthesis of Pt / TiO 2 The ionic liquid used is 1-ethyl-3-methylimidazolium chloride (EmimCl), as shown in formula (III).
[0051]
[0052] 6.25mg chloroplatinic acid hexahydrate (H 2 PtCl 6 ·6H 2 O) Dissolve in a mixture of 1.25g 1-ethyl-3-methylimidazolium chloride salt and 1.25g deionized water, add 1mL tetrabutyl titanate dropwise while vigorously stirring, transfer the above mixture into a 15mL autoclave , stirred at 150°C for 12h. After the reaction, the reaction kettle was naturally cooled to room temperature, and the reaction suspension was centrifuged to obtain a solid, which was fully washed with deionized water for 5 times, and vacuum-dried overnight at 80°C to obtain the final product.
[0053] The resulting product was characterized by X-ray diffraction, and the results were as follows: Figure 5 As shown, the obtained product is anatase phase TiO 2 (JCPDS card no. 21-127...
Embodiment 3
[0057] The Pt / TiO of this embodiment 2 The synthesis method is as follows:
[0058] Synthesis of Pt / TiO 2 The ionic liquid used is 1-ethyl-3-methylimidazolium bromide (EmimBr), as shown in formula (IV).
[0059]
[0060] 6.25mg chloroplatinic acid hexahydrate (H 2 PtCl 6 ·6H 2 O) Dissolve in a mixture of 1.25g 1-ethyl-3-methylimidazolium bromide and 1.25g deionized water, add 1mL tetrabutyl titanate dropwise while vigorously stirring, transfer the above mixture into a 15mL autoclave , stirred at 150°C for 12h. After the reaction, the reaction kettle was naturally cooled to room temperature, and the reaction suspension was centrifuged to obtain a solid, which was fully washed with deionized water for 5 times, and vacuum-dried overnight at 80°C to obtain the final product.
[0061] The resulting product was characterized by X-ray diffraction, and the results were as follows: Figure 8 As shown, the obtained product is anatase phase TiO 2 (JCPDS card no. 21-1272).
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com