Leaching method of refractory cobalt ore
A cobalt ore and leaching technology, applied in the field of leaching of refractory cobalt ore, can solve problems such as difficult impurity removal process, spillover environment, environmental pollution, etc., and achieve the effects of improving economic benefits and production, reducing environmental pollution, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The refractory cobalt ore used is high manganese cobalt oxide ore that is ground to a particle size of -200 mesh;
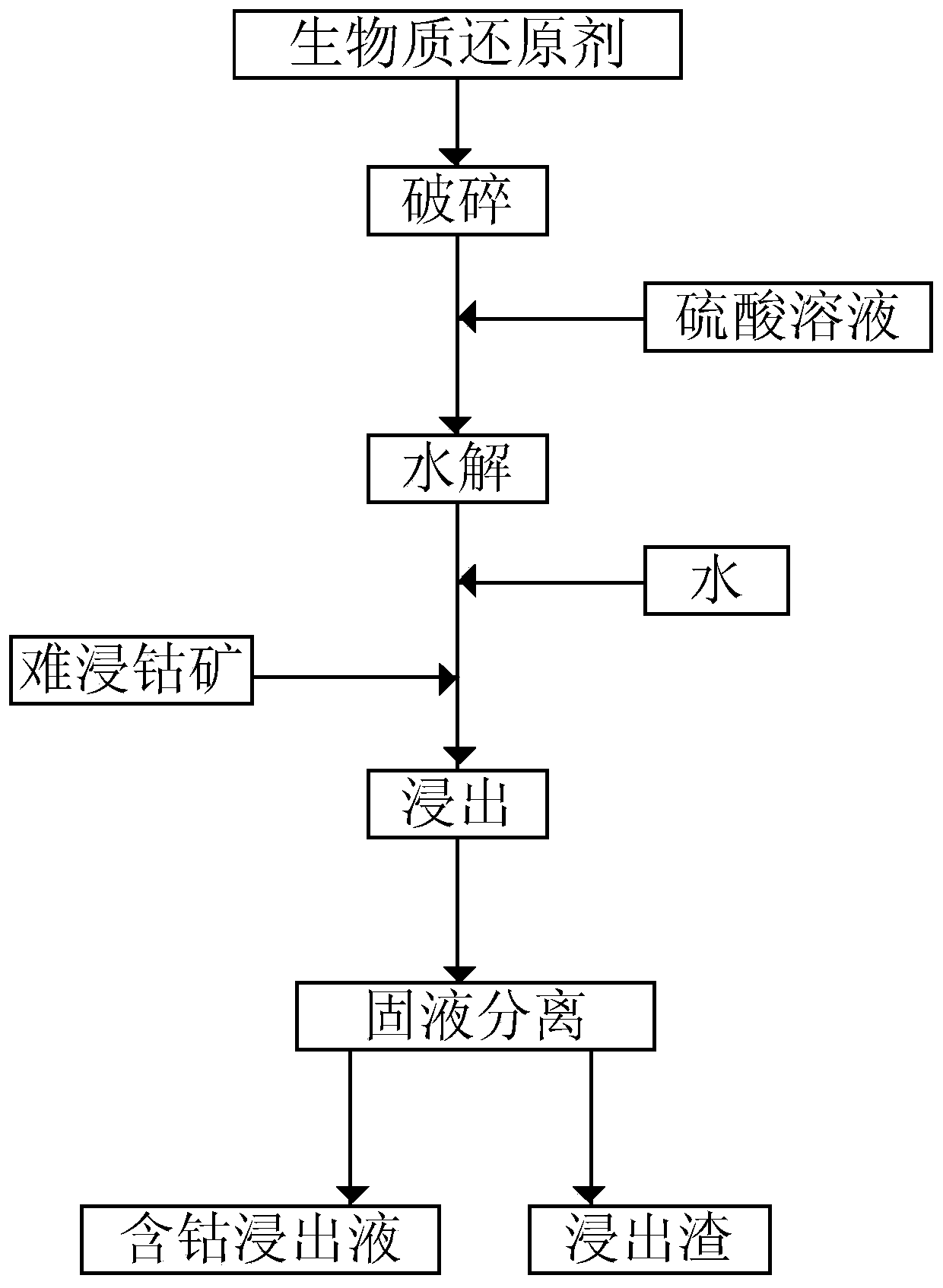
[0026] Process such as figure 1 shown;
[0027] Crush the biomass reducing agent to particle size -60 mesh, then add sulfuric acid solution for hydrolysis, the hydrolysis temperature is 40°C, and the hydrolysis time is 40 minutes to make hydrolyzed material; the biomass reducing agent is poplar, and the mass concentration of the sulfuric acid solution is 70%. The mass ratio of sulfuric acid solution and biomass reducing agent is 1;
[0028] Dissolve the hydrolyzed material in water, then put it into the refractory cobalt ore for leaching, the leaching temperature is 60°C, and the leaching time is 5h to obtain the leached material; the liquid-solid ratio of water to the hydrolyzed material is 3L / kg, and the hydrolyzed material and refractory cobalt The mass ratio of ore is 0.5;
[0029] Separating the leached material from solid to liquid to obtain a cob...
Embodiment 2
[0032] Method is with embodiment 1, and difference is:
[0033] (1) The hydrolysis temperature is 50° C., and the hydrolysis time is 20 minutes; the mass concentration of the sulfuric acid solution is 75%, and the mass ratio of the sulfuric acid solution and the biomass reducing agent is 1.5;
[0034] (2) The leaching temperature is 70°C, and the leaching time is 3 hours to obtain the leached material; wherein the liquid-solid ratio of water to the hydrolyzed material is 4L / kg, and the mass ratio of the hydrolyzed material to the refractory cobalt ore is 0.4;
[0035] (3) The concentration of Co in the cobalt-containing leaching solution is 8.59g / L, the leaching rate of cobalt is 99%, and the leaching rate of manganese is 98%.
Embodiment 3
[0037] Method is with embodiment 1, and difference is:
[0038] (1) The hydrolysis temperature is 45° C., and the hydrolysis time is 30 minutes; the mass concentration of the sulfuric acid solution is 80%, and the mass ratio of the sulfuric acid solution and the biomass reducing agent is 2;
[0039] (2) The leaching temperature is 80°C and the leaching time is 2h to obtain the leached material; wherein the liquid-solid ratio of water to the hydrolyzed material is 6L / kg, and the mass ratio of the hydrolyzed material to the refractory cobalt ore is 0.2;
[0040] (3) The concentration of Co in the cobalt-containing leaching solution is 6.45g / L, the leaching rate of cobalt is 99%, and the manganese leaching rate of manganese is 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com