Pig circovirus III-type virus-like particle and preparation method thereof
A porcine circovirus and virus-like technology is applied in the fields of botanical equipment and methods, biochemical equipment and methods, viruses, etc. It can solve the problems of poor immunogenicity of virus-like particles and is not suitable for large-scale production, and achieve good development and Application prospect, high safety, good immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
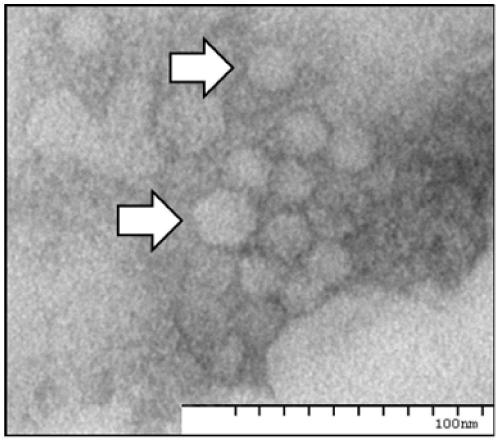
Examples
Embodiment 1
[0025] Example 1 Design and synthesis of porcine circovirus type III Cap protein gene
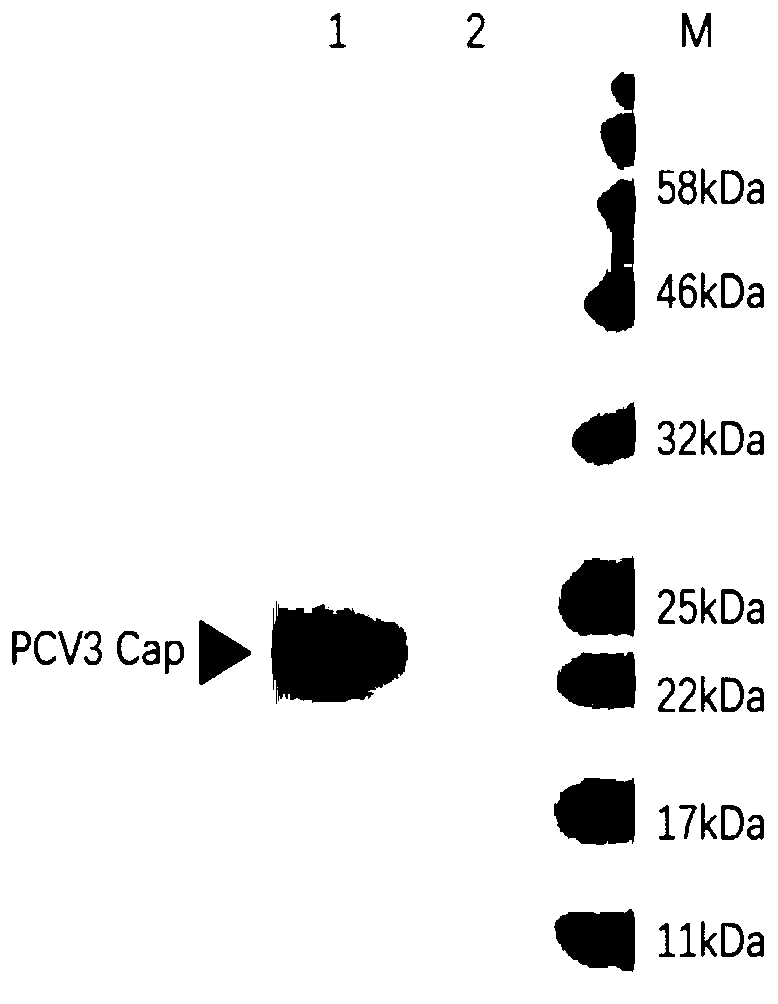
[0026] According to the published nucleotide sequence of porcine circovirus type III Cap protein (GenBank accession number: KX966193.1), add EcoRI enzyme before the start codon ATG of porcine circovirus type III Cap protein nucleotide sequence Cutting site: gaattc, add a His tag sequence after ATG, as shown in SEQ ID NO.2, add a Not I restriction site after the stop codon: gcggccgc, the optimally designed Cap protein gene sequence is shown in SEQ ID NO.1 Show. The sequence of the forward primer PCV3F for amplification of the nucleotide sequence of the Cap protein gene is shown in SEQ ID NO.3; the sequence of the reverse primer PCV3R is shown in SEQ ID NO.4. The gene was synthesized by Jinweizhi Biotechnology Co., Ltd., and cloned into the pUC57 vector, named pUC57-Cap.
Embodiment 2
[0027] Example 2 Construction and Identification of Recombinant Shuttle Plasmid pFB-Cap
[0028] Use restriction endonucleases EcoRI and NotⅠ to double digest pUC57-Cap and pFastBac 1 plasmids, react at 37°C for 2 hours, use 1% agarose gel electrophoresis to separate, and recover the Cap protein gene fragment and linearized pFastBac 1; Add 1 μL each of T4DNA ligase, T4Buffer, and linearized pFastBac 1 to 7 μL of the Cap protein gene fragment, mix gently, incubate at 25°C for 10 minutes, add the mixture to the just-thawed Trans5α competent cells, and incubate on ice for 20 minutes , heat shock at 42°C for 45s, immediately ice-bathed for 5min, activated at 37°C for 5min, spread evenly on the LB culture plate containing ampicillin (50μg / mL), culture upside down at 37°C for 12h, and obtain recombinant shuttle plasmid pFB-Cap colonies .
[0029] Pick a single colony, inoculate it in liquid LB medium containing ampicillin (50 μg / mL), culture at 37 °C, 220 rpm for 12 h with shaking,...
Embodiment 3
[0030] Example 3 Construction of rB-Cap recombinant bacmid and extraction of rB-Cap recombinant bacmid
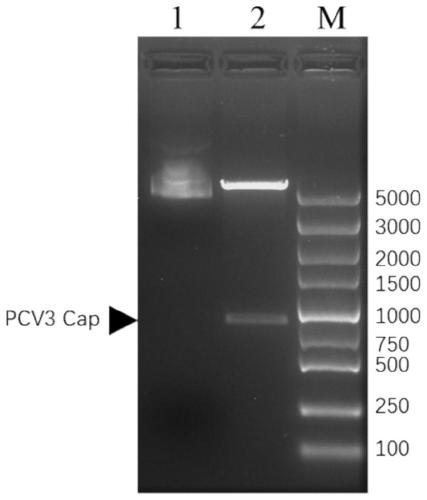
[0031] Take 1ng of the recombinant shuttle plasmid pFB-Cap in Example 2 and add it to the DH10Bac competent cells that have just been thawed on ice, mix gently and bathe in ice for 20min, heat shock at 42°C for 45s, immediately bathe in ice for 5min, use SOC without antibiotics The medium was shaken and activated at 37°C for 4 hours, and 80 μL was evenly spread on the medium containing kanamycin (50 μg / mL), tetracycline (10 μg / mL), gentamicin (7 μg / mL), X-Gal (100 μg / mL) , IPTG (40 μg / mL) on the LB blue-white screening plate, cultured upside down at 37°C for 48 hours, picked white colonies for PCR identification, and used the three-line method to streak the bacterial solution containing the target band on the above-mentioned LB blue-white screening plate , after inverting at 37°C for 36 hours, pick the white colony for PCR identification to obtain the rB-Cap recombinant vec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com