Polycarbosilane containing high-activity cross-linking groups and preparation method thereof
A technology of polycarbosilane and cross-linking groups, which is applied in the field of preparation of silicon carbide ceramic precursors, can solve the problems of long reaction time, residue, and difficult removal of catalysts, and achieve short reaction time, short time and few side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
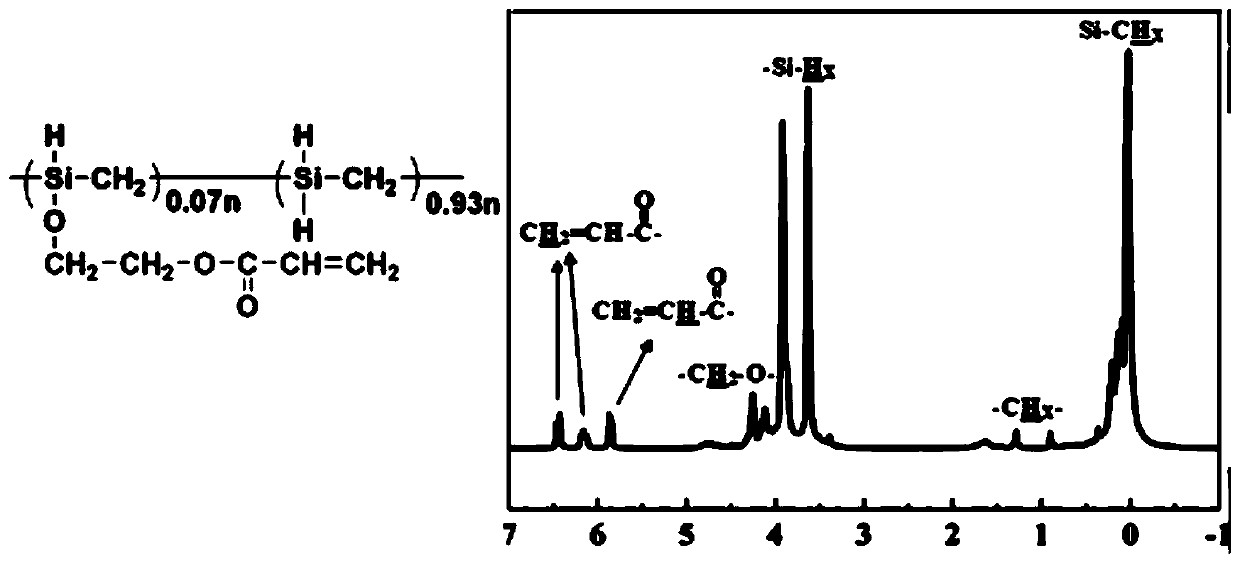
[0048] (1) Referring to the literature (J.Eur.Ceram.Soc., 2017; 37(10):3263–3270), the synthetic structural unit is mainly -SiH 2 -CH 2 - Liquid polycarbosilane. In an ice-water bath, add 4.4g of liquid polycarbosilane and 60ml of dry dehydrated n-heptane into the flask, and then 2 and concentrated HCl quantitatively produced Cl 2 (0.01mol) passed through saturated brine, concentrated sulfuric acid, and anhydrous calcium chloride successively, and then passed into a flask to stir and react for 30 minutes.
[0049] (2) Under an ice-water bath, add hydroxyethyl acrylate (0.01 mol) and triethylamine (0.025 mol), stir and react for 10 minutes, then centrifuge at high speed to remove the ammonium salt. Then add 5ml of acetonitrile, stir and centrifuge, remove the acetonitrile solution with a separatory funnel, then add 5ml of acetonitrile, stir and centrifuge, then remove the acetonitrile solution with a separatory funnel, the obtained n-heptane solution is concentrated under re...
Embodiment 2
[0055] Under an ice-water bath, 4.4 g of the liquid polycarbosilane synthesized in Example 1, 60 ml of dehydrated n-heptane and thionyl chloride (0.01 mol) were added into the flask. After stirring and reacting for 20 minutes, hydroxyethyl acrylate (0.01 mol) and triethylamine (0.025 mol) were added in an ice-water bath, and after stirring and reacting for 10 minutes, the ammonium salt was removed by high-speed centrifugation. Then add 5ml of acetonitrile, stir and centrifuge, remove the acetonitrile solution with a separatory funnel, then add 5ml of acetonitrile, stir and centrifuge, then remove the acetonitrile solution with a separatory funnel, the obtained n-heptane solution is concentrated under reduced pressure at room temperature to obtain Based liquid polycarbosilane.
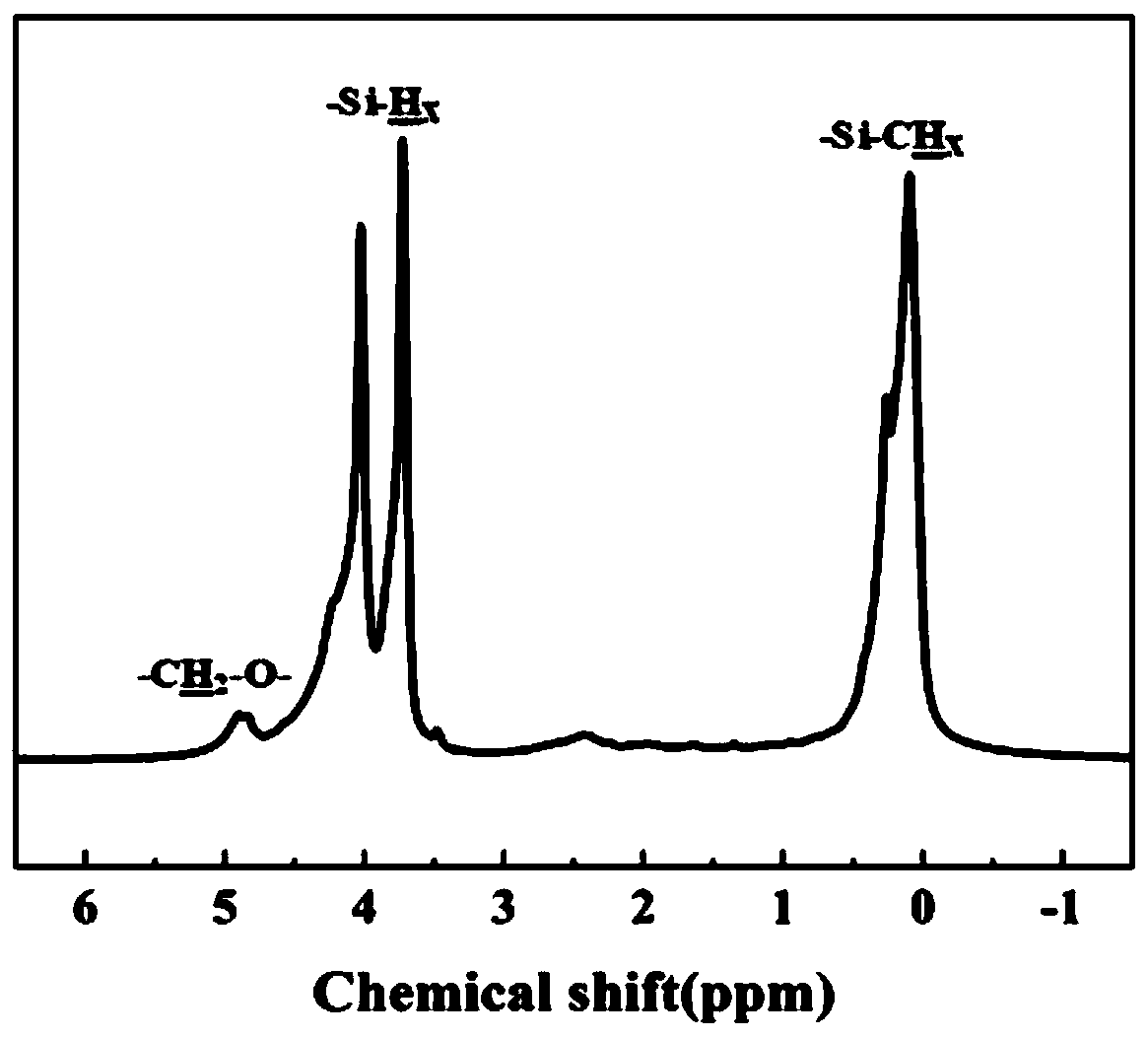
[0056] According to NMR, in the structure of the obtained liquid polycarbosilane containing acryloyloxy group, the molar content of structural units containing acryloyloxy group was 9%. Take 1 g of the...
Embodiment 3
[0058] Under an ice-water bath, 4.4 g of the liquid polycarbosilane synthesized in Example 1, 60 ml of dehydrated benzene and thionyl chloride (0.006 mol) were added into the flask. After stirring and reacting for 5 minutes, hydroxypropyl methacrylate (0.008 mol) and triethylamine (0.015 mol) were added under an ice-water bath, and after stirring and reacting for 5 minutes, the ammonium salt was removed by high-speed centrifugation. Benzene and unreacted triethylamine were distilled off under reduced pressure. Subsequently, add 80ml of cyclohexane and 5ml of acetonitrile, centrifuge after stirring, remove the acetonitrile solution with a separatory funnel, then add 5ml of acetonitrile, centrifuge after stirring, then remove the acetonitrile solution with a separatory funnel, and depressurize the obtained cyclohexane solution at room temperature Concentrate to obtain liquid polycarbosilane containing methacryloxy groups.
[0059] According to NMR, in the structure of the obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com