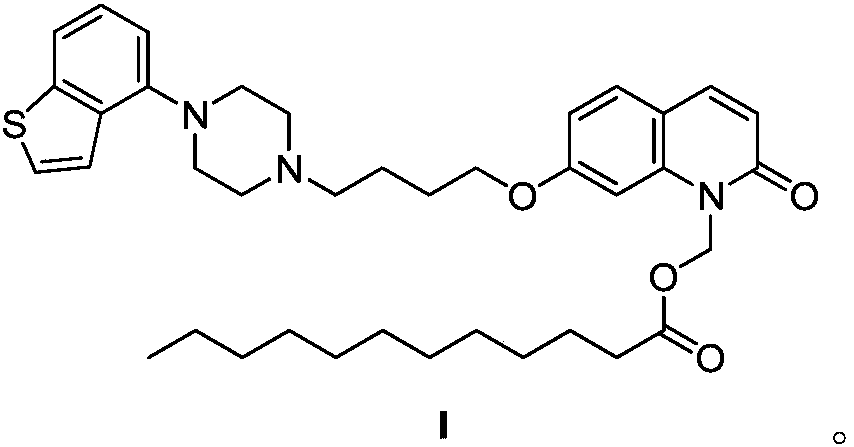
Crystal form A of brexpiprazole laurate, preparation method and application thereof
A technology of ebiprazole and laurate, applied in the field of medicinal chemistry, can solve the problems of low yield and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: the preparation of ebiprazole laurate
[0075]
[0076] Take 1.00 g of ebiprazole in a 50 mL three-necked flask, dissolve it with 20 mL of 1,4-dioxane until clear, and then add 0.28 g of NaH. After stirring at room temperature for 15 minutes, 1.14 g of chloromethyl laurate was added dropwise with an addition funnel, and 0.10 g of KI was added. The reaction solution was heated to 90° C., stirred for 2 h, then cooled to room temperature, and then quenched by pouring the reaction solution into a mixed solution of ethyl acetate and ice water. Finally, the mixed solution was transferred to a separatory funnel for separation, and the upper layer was washed twice with saturated sodium chloride solution, and then the remaining water was removed with anhydrous sodium sulfate, and about 1.50 g of crude ebiprazole laurate was obtained after spin-drying. 1 HNMR (400MHz, CDCl 3 ): δ7.61(d, J=9.5Hz, 1H), 7.54(d, J=8.0Hz, 1H), 7.40(dt, J=13.5, 7.1Hz, 3H), 7.26(t, J=...
Embodiment 2
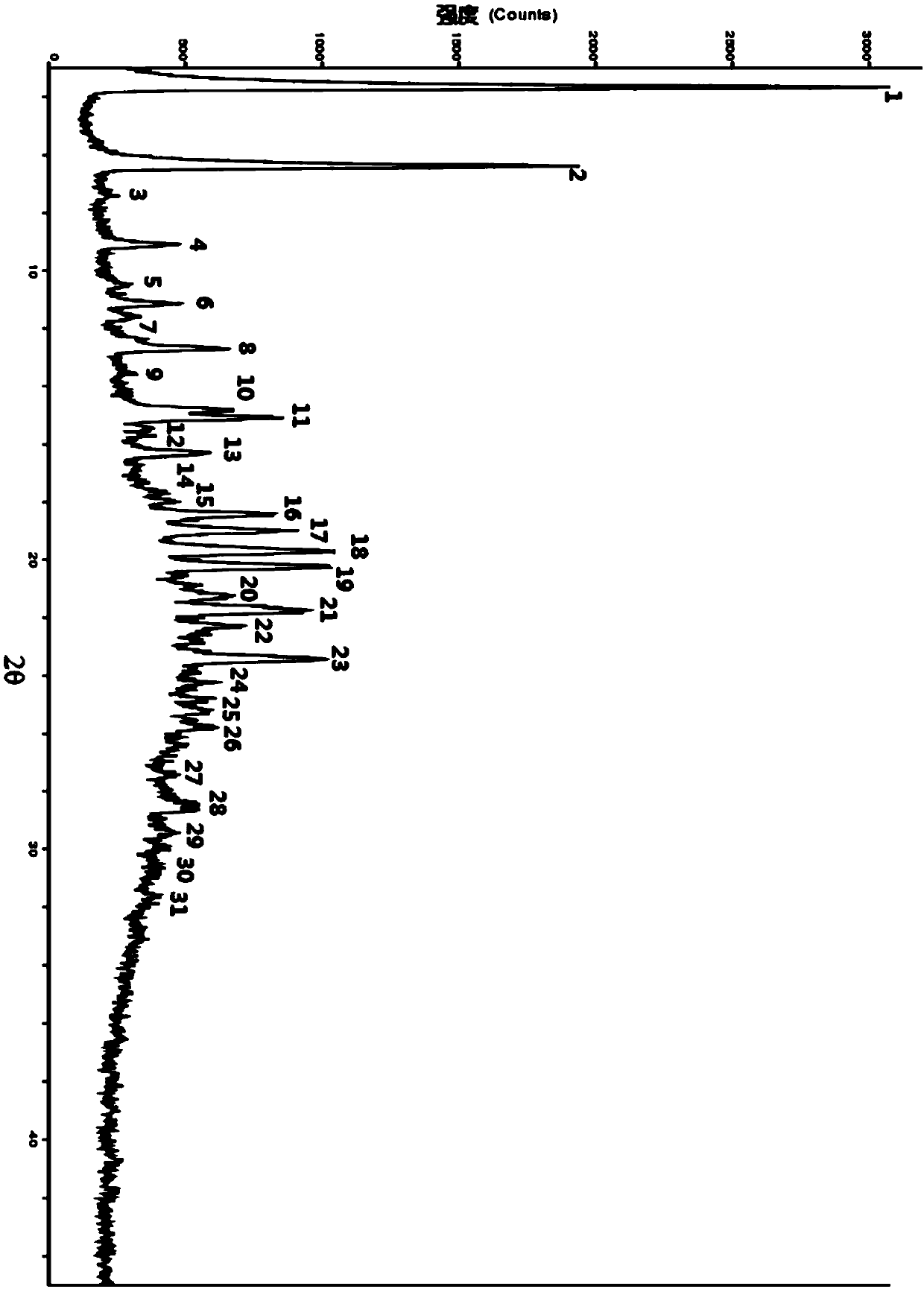
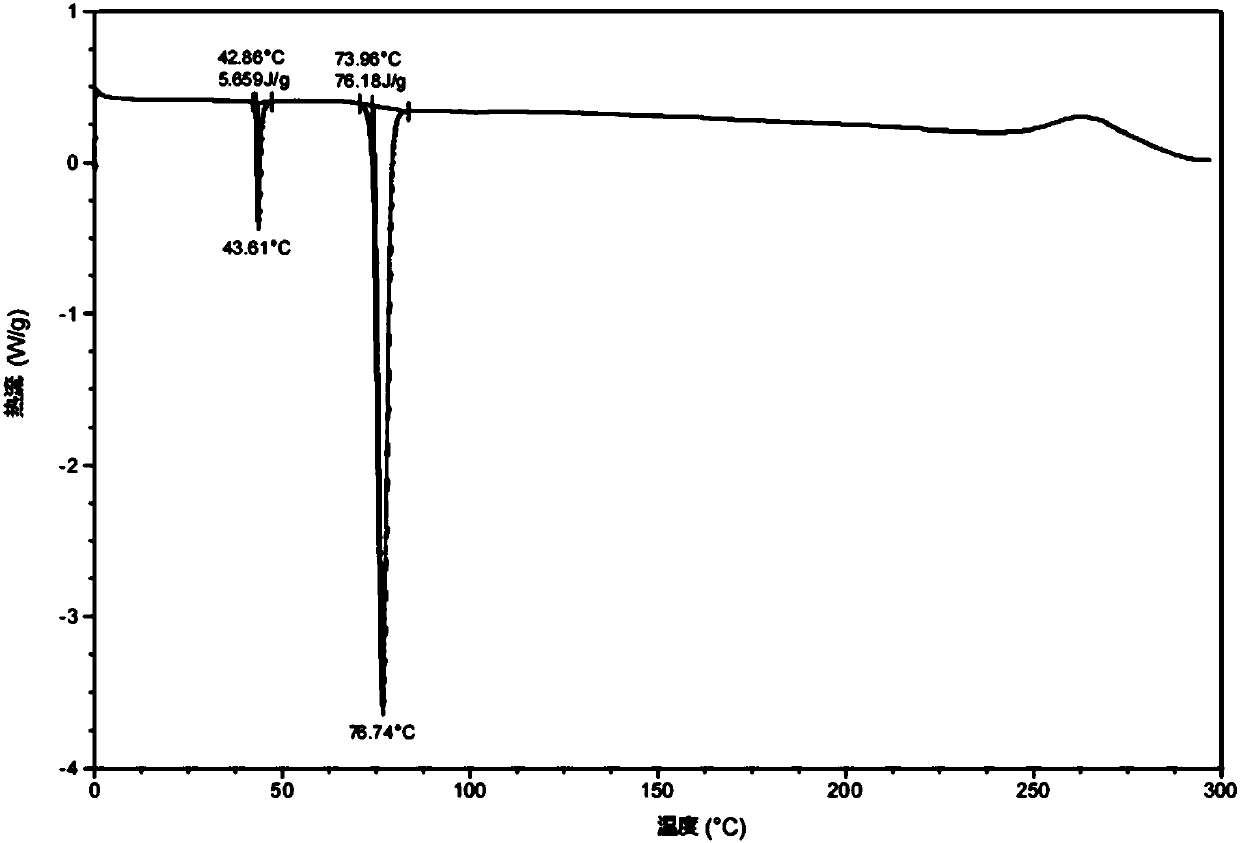
[0077] Example 2: Preparation of Form A of Ebiprazole Laurate
[0078]Take 1 g of ebiprazole laurate prepared in Example 1, and add 10 mL of ethyl acetate to form a solution. Slowly add 10 mL of n-hexane dropwise at a low temperature of -20°C and a stirring speed of 100 rpm, and keep stirring at low temperature for 2 h to gradually precipitate a white solid. The white solid was obtained by suction filtration, washed twice with 5 mL of n-hexane, and dried in a vacuum oven at -0.08 Mpa, 40°C for 2 hours to obtain the crystal form A of ebiprazole laurate with a yield of 84.7%. Its content was determined to be 99.62% by high performance liquid chromatography. Specific conditions for HPLC: Welch for chromatographic column LP-C18 (250*4.6mm, 5μm), column temperature 40°C; mobile phase A is pure acetonitrile, mobile phase B is 0.15% triethylamine aqueous solution (pH 3.50), mobile phase A:B is 90:10 ; The injection volume is 10μL; the flow rate is 1.2mL / min; the detection wavelen...
Embodiment 3
[0079] Example 3: Preparation of Form A of Ebiprazole Laurate
[0080] Take 1 g of ebiprazole laurate prepared in Example 1, and add 10 mL of ethyl acetate to form a solution. Slowly add 20 mL of n-hexane dropwise at a low temperature of -20°C and a stirring speed of 100 rpm, and keep stirring at low temperature for 2 hours to gradually precipitate a white solid. The white solid was obtained by suction filtration, washed twice with 5 mL of n-hexane, placed in a vacuum oven at -0.08 Mpa, and dried at 40°C for 2 hours to obtain crystalline form A of ebiprazole laurate with a yield of 85.7%. Its content was determined to be 99.83% by high performance liquid chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com