Cyclobutane dicarboxylic acid platinum complex, intermediate thereof, preparation method thereof, pharmaceutical composition and purpose
A technology of cyclobutane dicarboxylic acid and platinum complexes, which can be used in drug combination, preparation of sugar derivatives, pharmaceutical formulations, etc., and can solve the problems of drug resistance of toxic side effects, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
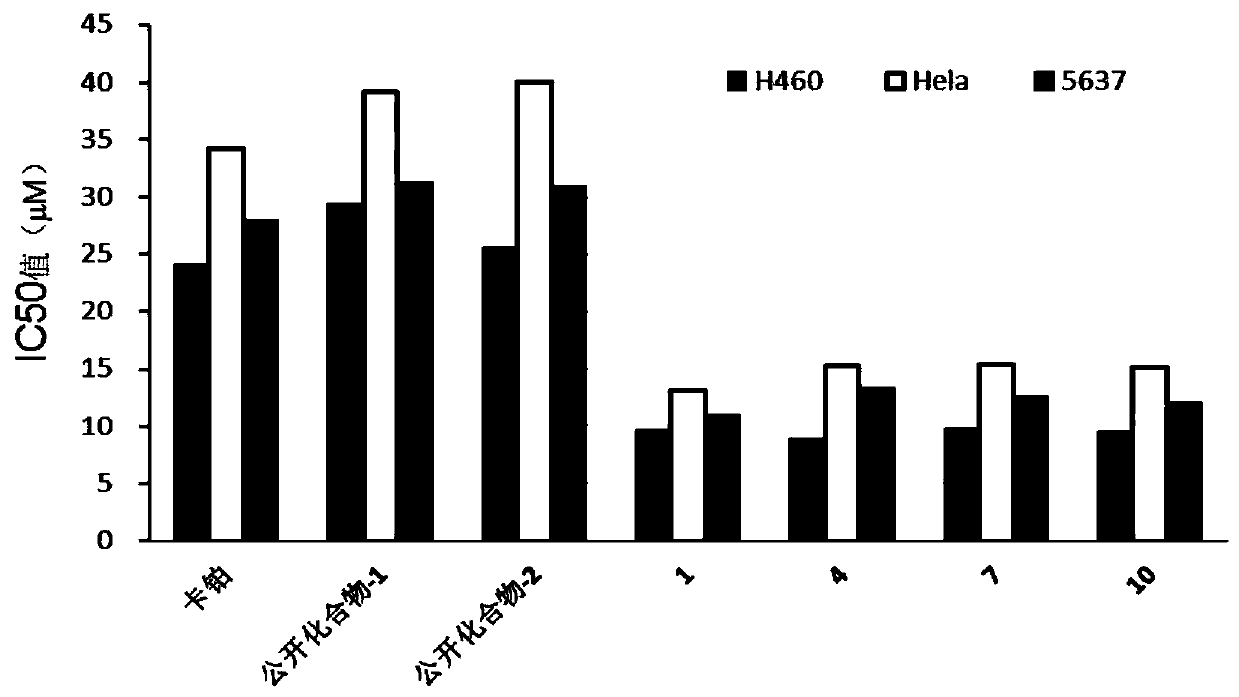
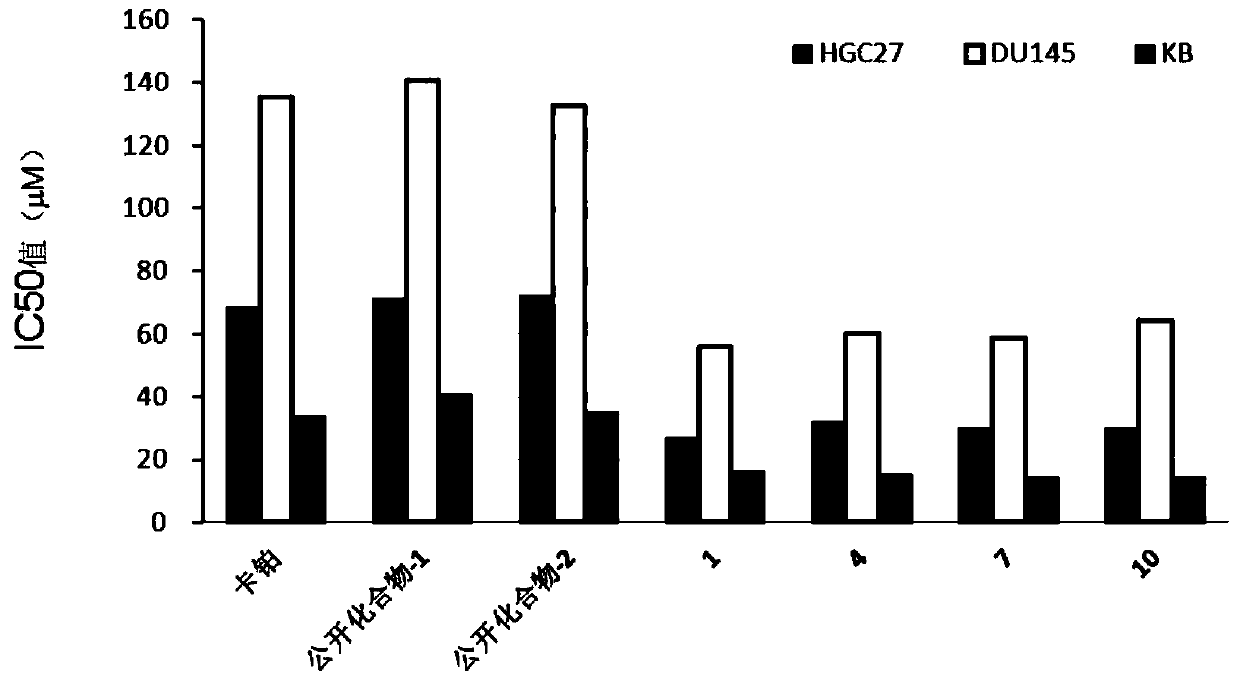
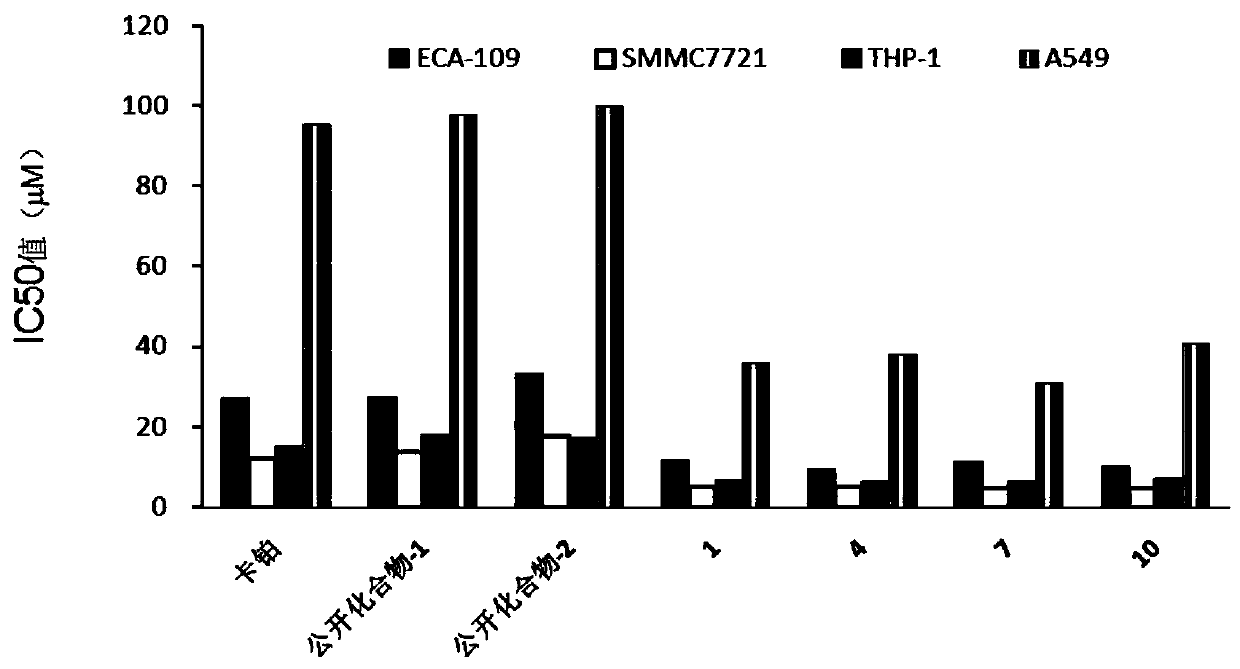
Image
Examples
Embodiment 1
[0109] Example 1: Preparation of cis-[trans-(1R,2R)-diaminocyclohexane]platinum(II)[3-(1-O-D-glucoside)cyclobutane-1,1-dicarboxylic acid]
[0110]
[0111] (1) Preparation of 1,1-ethyl dicarboxylate-3-acetoxy-cyclobutane
[0112]
[0113] 60% Sodium hydride (0.8g) was dissolved in a solution of N,N-dimethylformamide (15ml) in diethylmalonate (1.6g). The mixture was stirred at room temperature for 30 minutes. Then, a N,N-dimethylformamide solution (10 ml) containing 1,3-dibromo-2-carboxypropane (1.3 g) was added to the reaction solution, and the mixture was stirred at 80°C for 6 hours. The solvent was removed by rotary evaporation, and the residue was redissolved in a mixed solution of ethyl acetate (150ml) and saturated ammonium chloride (150ml), and the layers were separated. The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography (petroleum ether / ethyl acetate: 50 / 1) to obtain a ...
Embodiment 2
[0131] Example 2: Preparation of cis-[trans-(1R,2R)-diaminocyclohexane]platinum(II)[3-(1-O-D-mannoside)cyclobutane-1,1-dicarboxylic acid]
[0132]
[0133] (1) Preparation of ethyl 1,1-dicarboxylate-3-[2,3,4,6-O-acetyl-1-O-D-mannoside]cyclobutane
[0134]
[0135] 1,2,3,4,6-O-pentaacetyl-D-mannose (1.84g) was added to 1,1-dicarboxylate-3-hydroxycyclobutane (1.02g) at room temperature In the dichloromethane (20ml) solution, cooled to 0 ℃, the air in the flask was replaced with nitrogen, and the ether solution of boron trifluoride (98%, 1.19ml) was slowly added dropwise under the protection of nitrogen. The reaction was stirred at 0°C for 15 minutes, then slowly warmed to room temperature and stirred for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and simple purification was carried out by silica gel column chromatography (petroleum ether / ethyl acetate=5 / 1) to obtain 1.39 g of crude product.
[0136] 1 H NMR (400MHz, CDCl ...
Embodiment 3
[0144] Example 3: Cis-[trans-(1R,2R)-diaminocyclohexane]platinum(II)[3-(1-O-D-galactoside)cyclobutane-1,1-dicarboxylic acid] preparation
[0145]
[0146] (1) Preparation of ethyl 1,1-dicarboxylate-3-[2,3,4,6-O-acetyl-1-O-D-galactoside]cyclobutane
[0147]
[0148] 1,2,3,4,6-O-pentaacetyl-D-galactose (1.84g) was added to 1,1-dicarboxylate-3-hydroxycyclobutane (1.02g) at room temperature In the dichloromethane (20ml) solution, cooled to 0 ℃, the air in the flask was replaced with nitrogen, and the ether solution of boron trifluoride (98%, 1.19ml) was slowly added dropwise under the protection of nitrogen. The reaction was stirred at 0°C for 15 minutes, then slowly warmed to room temperature and stirred for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the reaction product was simply purified by silica gel column chromatography (petroleum ether / ethyl acetate=5 / 1) to obtain 1.55 g of a crude product.
[0149] 1 H NMR (400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com