Probe for dual-mode bio-imaging
A biological imaging, dual-modal technology, applied in the direction of using spectral diagnosis, using fluorescence emission for analysis, instruments, etc., can solve the problems of increasing Raman signal background, incompatibility of fluorescence and Raman microscopic imaging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
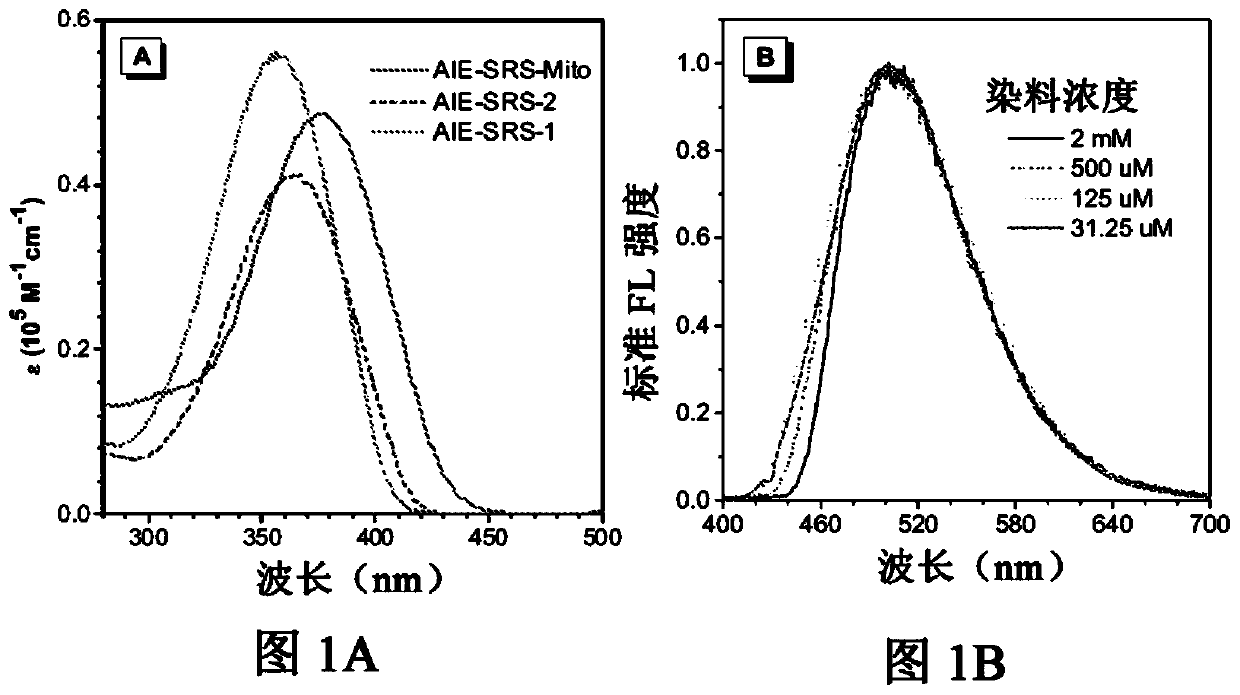
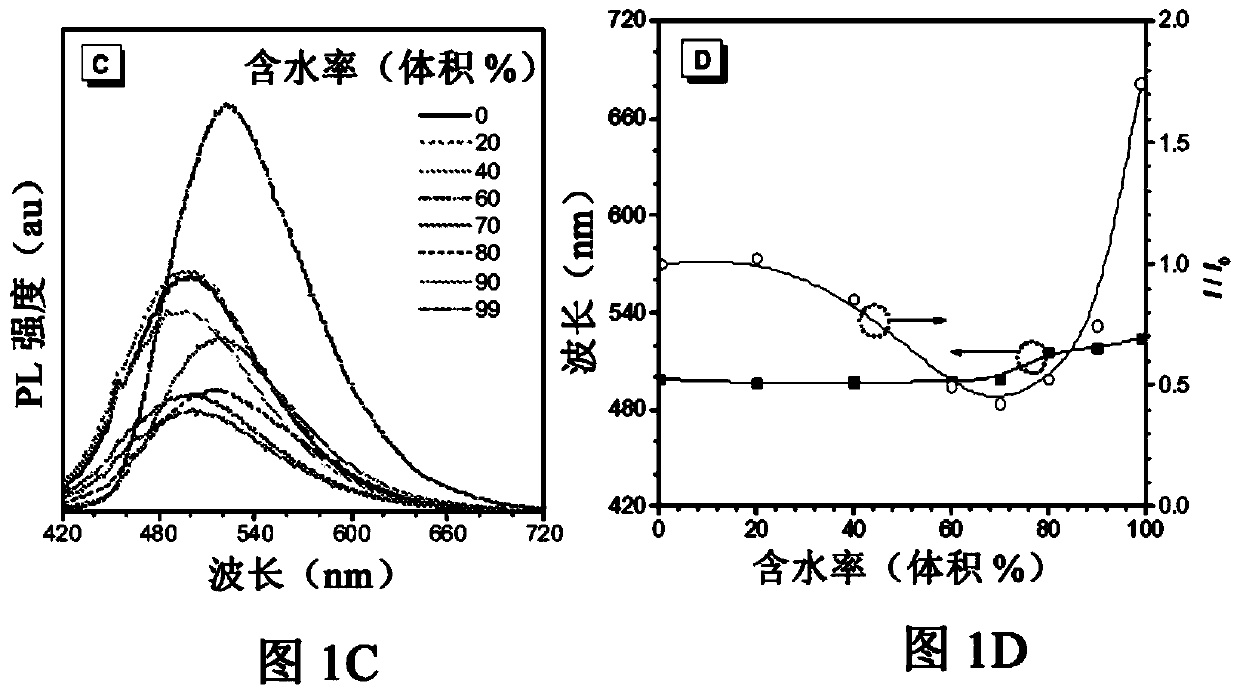
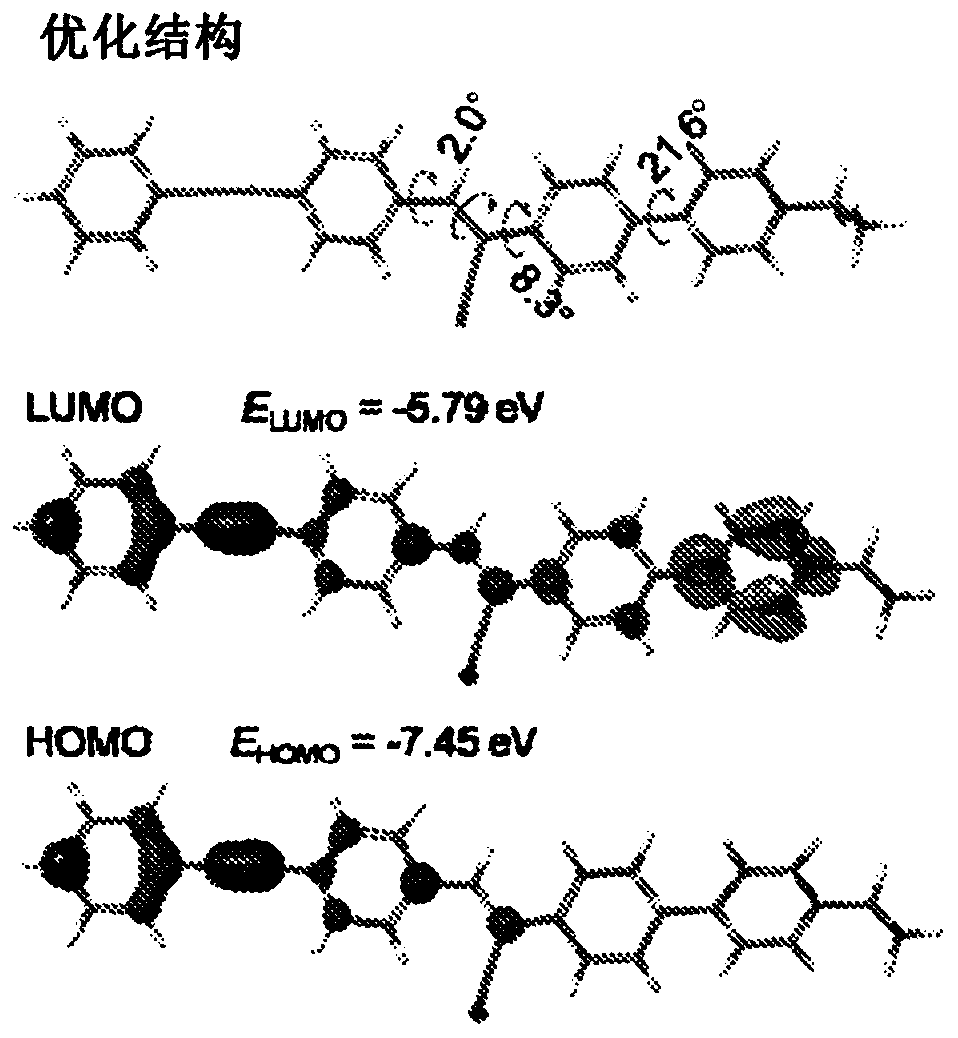
[0107] The method shown here is similar to the synthesis above using ASCP, except that the 4-(dimethylamino)benzaldehyde is replaced by commercially available 4-(phenylethynyl)benzaldehyde. As shown in the Alternative Reaction Schemes, the preparation of the compounds of the present invention may comprise the following sequential steps: Knoevenagel condensation, Suzuki coupling and alkylation. The structures of AIE-SRS-Mito and its synthetic precursors (AIE-SRS-1 and AIE-SRS-2) were confirmed by 1HNMR, 13C NMR and high-resolution mass spectrometry with satisfactory results.
[0108] Identify target of interest
[0109] According to one embodiment, one or more compounds of the invention may be contacted with a target cell to identify the target of interest in the target cell, eg, to detect the presence or absence of the target of interest. Targets of interest can be identified by imaging methods such as fluorescence microscopy and / or Raman microscopy. Targets of interest ma...
Embodiment 1
[0120] Synthesis of AIE-SRS-1
[0121] In a 100 mL round bottom flask, 4-(phenylethynyl)benzaldehyde (206 mg, 1 mmol) and 4-bromophenylacetonitrile (196 mg, 1 mmol) were dissolved in 40 mL of ethanol solution to form a mixture. Sodium hydroxide (100 mg) was added to the mixture. After stirring at 50° C. for 5 hours, the resulting pale yellow precipitate was filtered and washed with cold ethanol. The product was dried and weighed. Yield: 95%. 1 H-NMR (400MHz, d 6 -DMSO)δ(ppm)8.13(s,1H),8.00(d,2H,J=8.0Hz),7.74-7.71(m,6H),7.60-7.58(m,2H),7.46-7.45(m, 3H). 13 C NMR (100MHz, d 6 -DMSO) δ (ppm) 142.4, 133.6, 133.0, 132.1, 131.9, 131.5, 129.5, 129.2, 128.8, 127.9, 124.4, 122.8, 121.9, 117.5, 109.8, 91.8, 89.0. MS (MALDI-TOF): AIE-SRS-1 (C 23 h 14 Calculated for BrN: 383.0310, found: 383.0309.
Embodiment 2
[0123] Synthesis of AIE-SRS-2
[0124] Compound 1 (192 mg, 0.5 mmol), 4-pyridylboronic acid (74 mg, 0.6 mmol), potassium carbonate (172 mg, 1.25 mmol) and Pd(PPh 3 ) 4 (10 mg, 0.01 mmol) in total of 10 mL of aqueous solution and 3 mL of water were added to a 100 mL two-neck round bottom flask equipped with a condenser to form a mixture. The mixture was stirred and heated to reflux overnight. After cooling to room temperature and evaporating THF, the mixture was extracted three times with dichloromethane (DCM). The organic phase was collected, washed with water and dried over anhydrous magnesium sulfate. After solvent evaporation, the crude product was purified by silica gel column chromatography using DCM / MeOH as eluent. Yield: 99%. 1 H NMR (400MHz, CDCl 3 )δ (ppm) 8.71 (d, 2H, J = 4.4Hz), 7.93 (d, 2H, J = 8.4Hz), 7.82 (d, 2H, J = 8.4Hz), 7.74 (d, 2H, J = 8.4 Hz), 7.63 (d, 2H, J=8.4Hz), 7.60-7.54 (m, 5H), 7.39-7.36 (m, 3H). 13 C NMR (100MHz; CDCl 3 )δ (ppm) 150.7, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com