Lithium carbonate preparation method
A lithium carbonate and lithium bicarbonate technology, applied in lithium carbonate;/acid carbonate and other directions, can solve the problems of inability to prepare lithium carbonate, low extraction efficiency and high production cost, and achieve abundant raw materials and high extraction rate. , to avoid the effect of acid corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
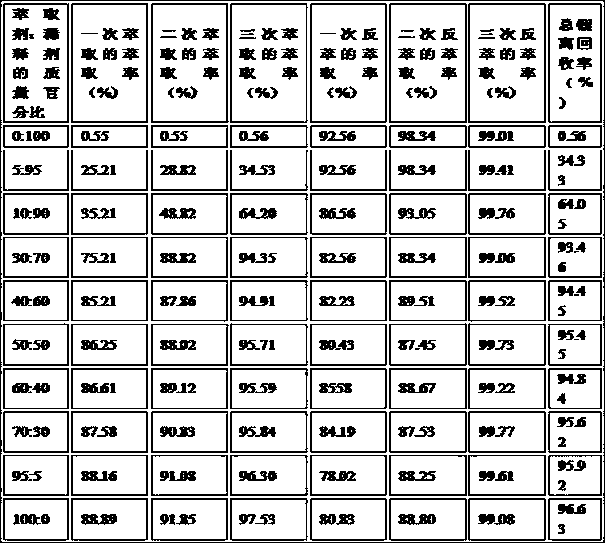
[0012] Examples of the present invention provide a lithium carbonate preparation method. This method uses small water -soluble, low toxicity, low price, and an extraction organic phase with a high extraction rate for lithium ion to form an alkaline extraction system with lithium solution. The extraction rate of lithium ions in lithium -containing solutions can avoid acid corrosion of production equipment; at the same time, one or its arbitrary combination of carbon dioxide into pure water, alkaline metal salt or ammonium saline solution is used to obtain The water solution as an anti -extraction solution, the acidity of the anti -extraction system formed by the organic phase of the lithium -containing organic phase is less than 6 ~ 9mol / L, and can directly convert the lithium ion in the lithium -containing organic phase in an alkaline environment to , Heat the lithium bicarbonate solution collected by lithium ions, which can make lithium bicarbonate crystals in lithium bicarbonate...
Embodiment 1
[0037] Examples of the present invention provide a method of preparing lithium carbonate, including the following steps:
[0038] The lithium -containing solution is selected to contain 0.26g / L salt lake brine, and its pH value is 9.
[0039] Extractive stage: separate extraction agents (10ml of benzolrazone, 10ml of triangular oxidation), 20ml of triangular oxidation, and diluent (ordinary kerosene) mixed in the division of liquid funnel, and then add 390ml of salt brine, the control temperature is in the control temperature in At 5 ° C, the water phase and lithium -containing organic phase are separated after the oscillation L in minutes. Repeat the above extraction operation three times.
[0040] Anti -extraction phase: The anti -extraction solution uses carbon dioxide and 2%sodium bicarbonate aquatic solution with carbon dioxide and mass concentration. The above extraction phase is separated from the iconic organic phase and brine mixed with a brine of 390ml, and then the pres...
Embodiment 2
[0044] Examples of the present invention provide a method of preparing lithium carbonate, including the following steps:
[0045] The lithium -containing solution is concentrated in salt lake containing 2.2g / L, and its pH value is 11.
[0046] Extractive stage: separate extraction agents (5ml of phenyl dexilide, 5ml oxygen oxidation) 10ml, diluent (ordinary kerosene) 650ml mixed in the division of functions, and then add salt lake concentrated brine for 2000ml. The control temperature is in the control temperature. 25 ° C. After oscillation L0 minutes, the water phase and lithium -containing organic phase are separated, and the above extraction operations are repeated three times.
[0047] Anti -extraction phase: The anti -extraction solution uses carbon dioxide and 2%potassium carbonate aquatic solution with carbon dioxide and mass concentration. The lithium organic phase obtained by the above extraction phase is separated from the 2000ml anti -extract, and then the pressure is 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com