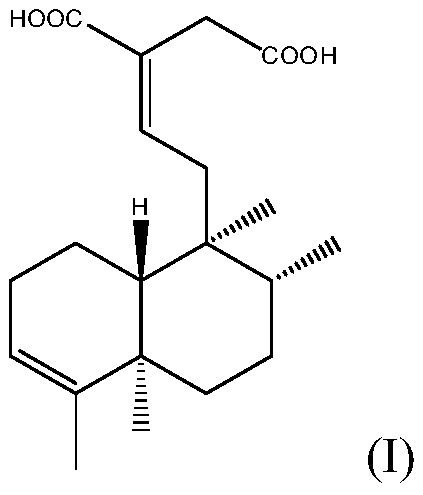
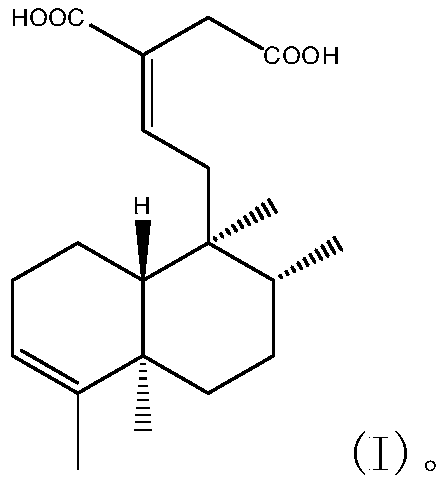
Selective beta1-adrenergic receptor and application thereof in cardiovascular disease medicines
A cardiovascular and drug technology, applied in the field of medicine, can solve the problems of loss of specificity, adverse effects of the respiratory system, etc., and achieve stable quality, excellent β1-adrenergic receptor inhibitory activity, and good physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 The beneficial effects of active compounds of the present invention are further set forth below through in vitro pharmacological activity experiments, but this should not be interpreted as only having the following beneficial effects on the compounds of the present invention
[0034] The experimental rats were Wistar rats, male or female, weighing 300±25 g, provided by the Experimental Animal Center of Peking University Health Science Center.
[0035] Materials: The β1 receptor membrane protein is prepared from the heart tissue of Wistar rats, and the β2 receptor membrane protein is prepared from the lung tissue of Wistar rats. The preparation method can be prepared with reference to the method of Williams, LT (referring to Williams, LT: Science, 1976, 192:791). Specifically, rats were decapitated and bled, and the rat heart and lung tissues were quickly taken out and placed in pre-cooled centrifugation buffer at 0-4°C, and the envelope was peeled off. Then ad...
Embodiment 2
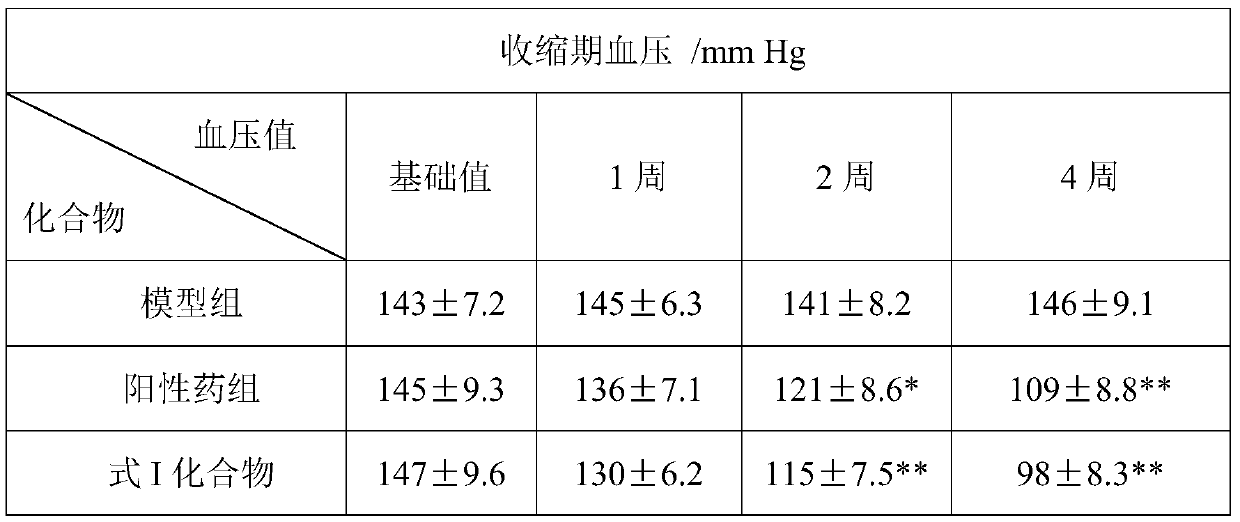
[0040] The effect of embodiment 2 formula I compound on SHR spontaneously hypertensive rat model
[0041] Based on the blocking results of the compound of formula I on β-receptor, we further used the model of spontaneously hypertensive rats (SHR rats) to evaluate the antihypertensive ability of the compound of formula (I) of the present invention.
[0042] Experimental method: 16-week-old SHR rats, male or female, were randomly divided into 4 groups: model group, positive drug group, and drug administration group, with 8 rats in each group. Rats in each group were reared in a conventional environment, free to drink water and ingest food, and began to administer intragastric administration after 3 days of feeding. The positive drug group was given atenolol at 5 mg / kg, the drug group was given the compound of formula I at 5 mg / kg, and the model group The same amount of normal saline was given once a day for 4 consecutive weeks.
[0043] Blood pressure measurement: The blood pre...
Embodiment 4
[0060] Embodiment 4 comprises the preparation of the pharmaceutical composition of formula (I) compound active ingredient
[0061] The pharmaceutical composition of this embodiment contains the following components by mass:
[0062]
[0063] The composition is prepared according to the following steps:
[0064]1) sieve the compound of formula (I) and cornstarch and mix into a homogeneous mixture;
[0065] 2) Granulate the mixture with water and allow to dry;
[0066] 3) the dried granules are mixed with sorbitol and calcium stearate to obtain lubricated granules;
[0067] 4) Compress the lubricated granules into tablet cores, each tablet core containing 25 mg of the compound of formula (I).
[0068] 5) The tablet core was coated with a sugar coating containing 2 wt% gum arabic / 77 wt% refined sugar / 20 wt% calcium sulfate / 1 wt% sodium carboxymethylcellulose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com