Preparation method of amorphous cefditoren pivoxil composition
A technology for cefditoren pivoxil and cefditoren axetil, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as poor crystal stability, difficult solvent drying, influence on preparation preparation, etc., and achieves shortening drying time, enhancing bioavailability, avoiding The effect of mixed crystal results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
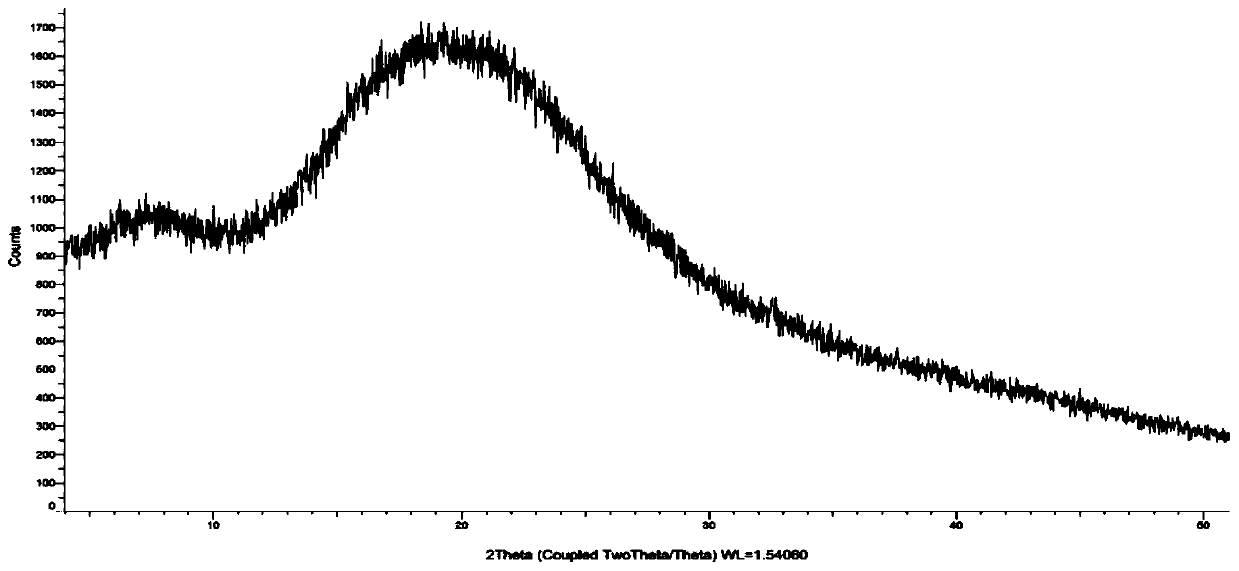
[0028] Suspend 50g of cefditoren axetil in 500ml of dichloromethane and acetone (volume ratio: 1:1), raise the temperature to 40°C and stir for 0.5h until completely dissolved, add 0.5g of hypromellose, stir for 5min until the hypromellose The powder is evenly dispersed, spray-dried, the air inlet temperature is 50-60°C, the atomization frequency is 35HZ-40HZ, and the outlet air temperature is 30-35°C. Collect the solid powder and send it to XRD for inspection. The spectrum is as follows figure 1 (no diffraction angle), confirmed to be amorphous.
Embodiment 2
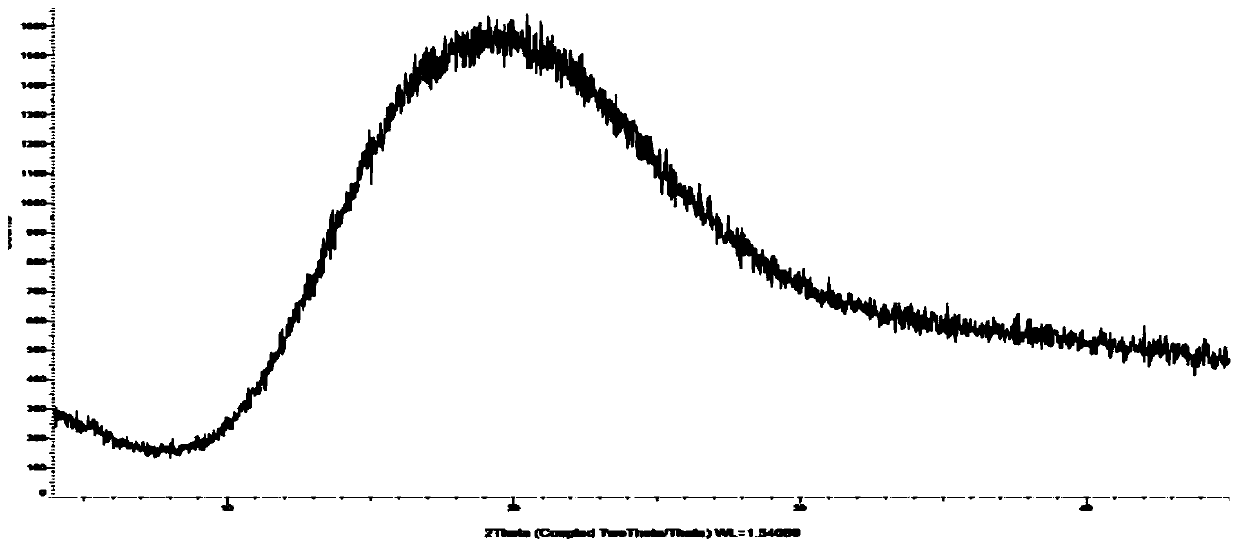
[0030] Suspend 20g of cefditoren axetil in 300ml of chloroform, acetonitrile, and ethanol (2:1:1), raise the temperature to 35°C and stir for about 0.5h-1h until completely dissolved, add 0.5g of hypromellose, and stir for 5min to The hypromellose is uniformly dispersed, spray-dried, the air inlet temperature is 65-70°C, the atomization frequency is 40-50HZ, and the outlet air temperature is 30-40°C. Collect the solid powder and submit it for XRD inspection. The spectrum is as follows figure 2 (no diffraction angle), confirmed to be amorphous.
Embodiment 3
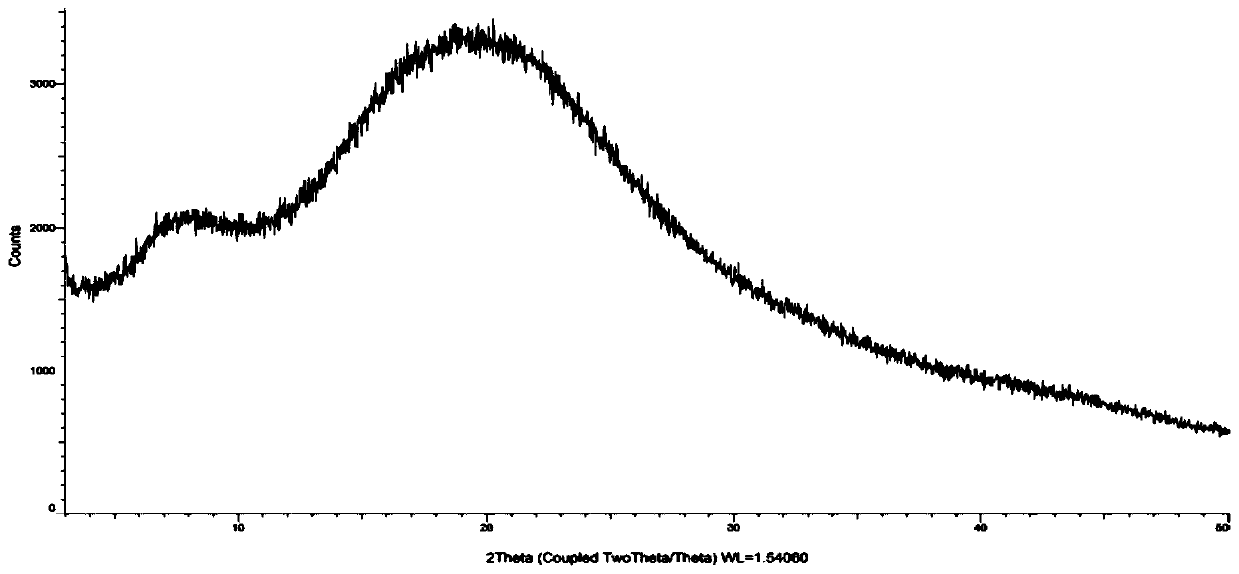
[0032] Suspend 50g of cefditoren axetil in 500ml of dichloromethane and acetone (volume ratio: 1:1), heat up to 40°C and stir for 0.5h until completely dissolved, add 0.25g of povidone k30, stir for 5min until it is uniformly dispersed, spray Drying, the inlet air temperature is 50-60°C, the atomization frequency is 30HZ-40HZ, the outlet air temperature is 30-35°C, collect the solid powder and submit it for XRD inspection, the spectrum is as follows image 3 (no diffraction angle), confirmed to be amorphous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com