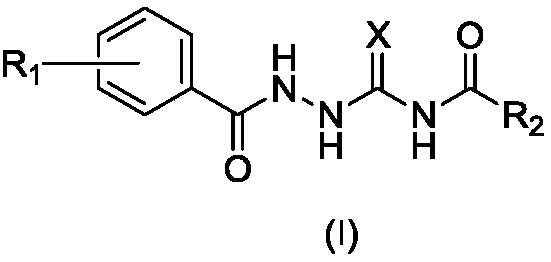
1-substituted benzoyl-4-fatty acylsemicarbazide derivative, preparation method thereof, and use thereof as antibacterial medicine
A technology of aliphatic acylsemicarbazides and propionyl thiosemicarbazides, which is applied in the field of preparation of 1-substituted benzoyl-4-fatty acylsemicarbazide derivatives, and can solve the problem of weak activity and 1-aroyl-4-lipids Acylthiosemicarbazide derivatives have few studies and no antibacterial activity, etc., to achieve a strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: the synthesis of compound 10
[0051]
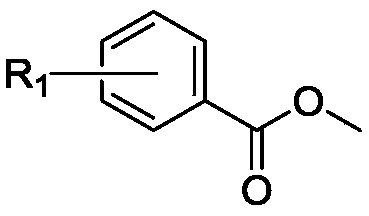
[0052] Add 25g (164mmol) of methyl 4-hydroxybenzoate and 34g (246mmol) of potassium carbonate and 600ml of acetone into a 1000ml flask, heat to 60°C, then add 19.4ml of 1-bromobutane to it, after the reaction is complete , remove the reaction solution under reduced pressure, add 300ml water to dissolve the product therein, extract 3 times with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter, and spin the filtrate to obtain 4-butoxybenzoic acid methyl ester 33.9 g (yield: 99%).
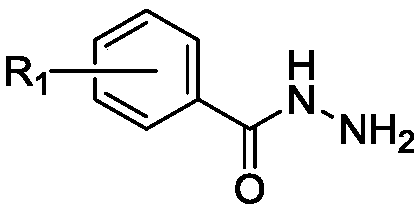
[0053] Add 33.9g (163mmol) of methyl 4-butoxybenzoate, 150ml of hydrazine hydrate (80%) and 500ml of methanol into a 1000ml three-necked flask, heat to reflux at 70°C, and spin the reaction solution to obtain a white Solid, washed the white solid three times with water, and filtered to finally obtain 30 g of 4-butoxybenzoic hydrazide (yield: 91%).
[0054]Add 8.64g (89mmol) potassium thiocyanate and 2...
Embodiment 2
[0058] Embodiment 2: the anti-bacillus subtilis activity assay of compound
[0059] 1. Preparation of test bacteria solution
[0060] Take 10g of sodium chloride, 10g of peptone, and 5g of yeast extract, add deionized water to make up to 1000mL, adjust the pH to 7.0, subpackage, and make LB medium after sterilization. Inoculate Bacillus subtilis (Bacillus subtilis CMCC 63501) into 20 mL of LB medium, and culture in a 37°C incubator with shaking at a speed of 200 rpm for 12 hours. When the clarified LB medium becomes turbid, it indicates that the bacterial proliferation is obvious and the growth is vigorous. At this time, the bacterial solution was diluted with new LB liquid medium to make its OD600 value between 0.3 and 0.5, and then diluted 105 times with new LB medium again as the test bacterial solution.
[0061] 2. Determine the minimum inhibitory concentration
[0062] Take a clean and sterile 96-well cell culture plate, add 200 μL of the prepared test bacteria solutio...
Embodiment 3
[0064] Example 3: Determination of the inhibitory activity of compounds on clinical common Gram-positive bacteria
[0065] 1. Measurement method
[0066] According to the guidelines for drug susceptibility testing issued by CLSI, it was determined by agar dilution method. (CLSI.Methods fordilution antimicrobial susceptibility tests for bacteria that growaerobically; approved standard-ninth edition.CLSI document M07–A9.Wayne, PA: Clinical and Laboratory Standards Institute; 2012.)
[0067] 2. Types of strains to be tested
[0068] Select 14 species of 4 types of Gram-positive bacteria common in clinical practice, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant surface Staphylococcus (MRSE), and vancomycin-resistant enterococcus (VRE) strains were used as the tested strains.
[0069] 3. Operation steps
[0070] After configuring the MH agar medium (pH 7.2-7.4) according to the product instructions, dilute it with a 2-fold gradient of the M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com