Purification method of virus-like particles of porcine parvovirus and application of purification method
A technology of parvovirus and purification method, which is applied to the preparation methods of peptides, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of low added value of products and increased production costs, and achieves the effect of being beneficial to production operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation process of embodiment 1 porcine parvovirus semi-finished product
[0023] The nucleotide sequence encoding the porcine parvovirus virus-like particle gene is connected to the baculovirus to construct a recombinant baculovirus seed, and the virus seed infects insect cells (Sf9 cells), and the culture conditions of Sf9 cells are 27°C, 140rpm, 96h~ 120h. When the cells were lysed to release the virus, the venom was collected and the porcine parvovirus virus-like particles were inactivated by binary ethylenimine (BEI).
Embodiment 2
[0024] Example 2 Purification of porcine parvovirus virus-like particles
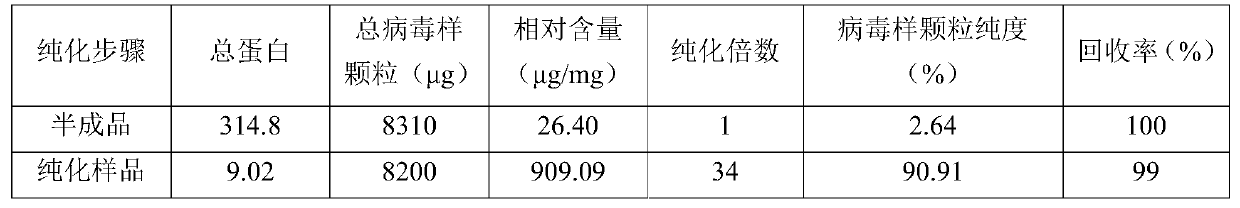
[0025] The inactivated venom is clarified to remove the precipitate, the pH of the semi-finished product is adjusted to 5.5-6.0 with 2% acetic acid, and the semi-finished product before purification of porcine parvovirus virus-like particles is obtained.
[0026] 1) weak anion exchange chromatography
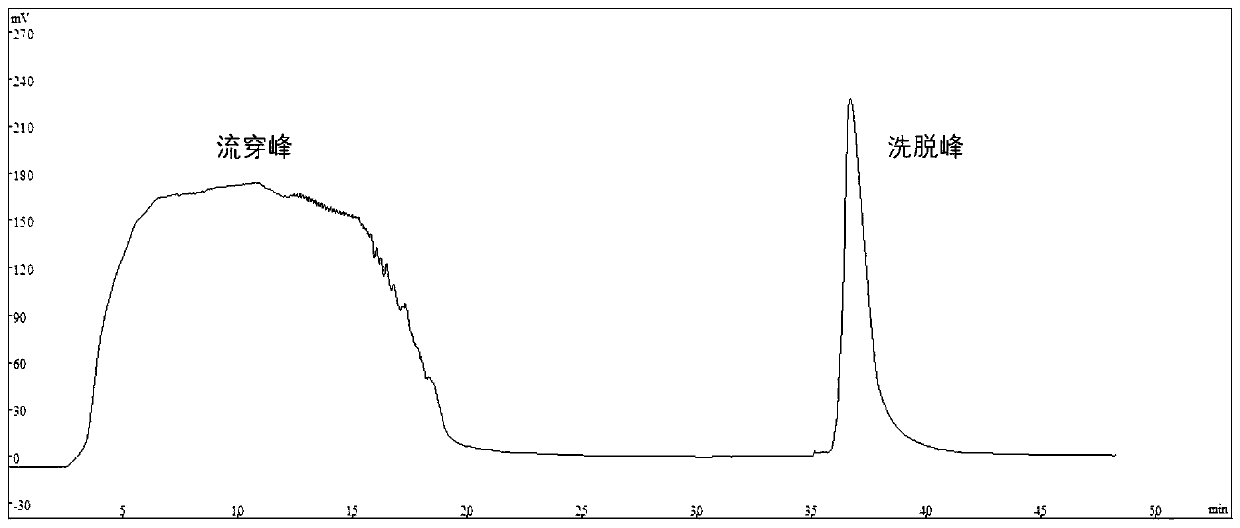
[0027] The pretreated semi-finished product was added to a weak anion exchange column (DEAE Focusose 6XL, Wuhan Huiyan Biotechnology Co., Ltd.) equilibrated with sodium acetate buffer A (20mM sodium acetate, 0.1M NaCl, pH=5.5), Use sodium acetate buffer B (20mM sodium acetate, 1.0M NaCl, pH=5.5) for elution, the elution volume is 3 column volumes, the flow rate is 2.0mL / min, and the peak components are collected, and the weak anion exchange Chromatograms such as figure 1 shown.
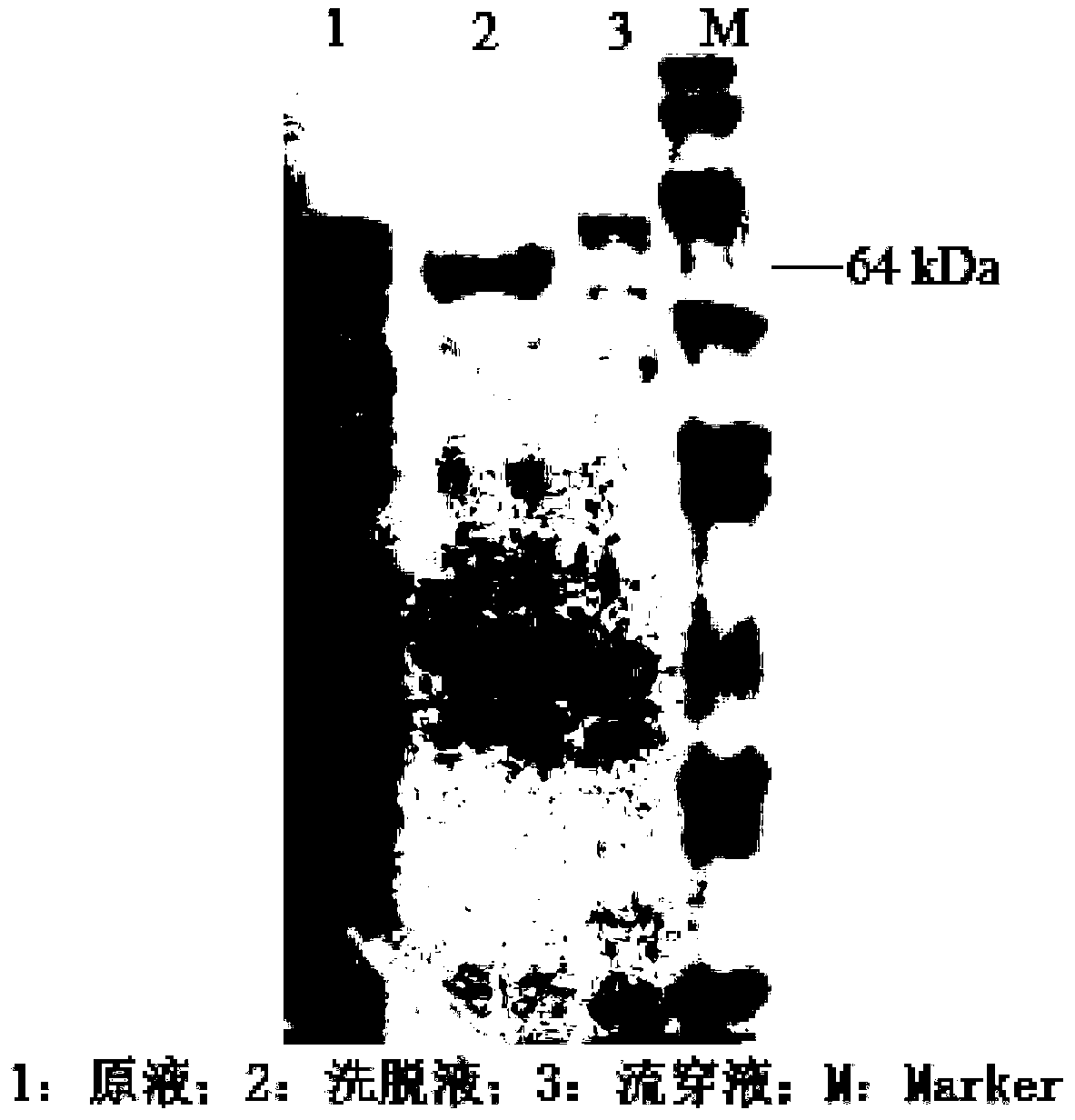
[0028] 2) Determination of active protein components by polyacrylamide gel electrophoresis (SDS-PAGE) Take 80 μL of samples ...
Embodiment 3
[0050] Example 3 Safety Test of Vaccines Containing Porcine Parvovirus Virus-like Particles
[0051] 1. The same amount of adjuvant was added to the samples before and after the purification of the experimental vaccine porcine parvovirus virus-like particles to prepare the vaccine. The vaccine batch before purification was defined as VP2-01, and the vaccine batch after purification was defined as VP2-02.
[0052] 2. Test piglets were purchased from Yunli Animal Husbandry Co., Ltd., Dantu District, Zhenjiang City, and all of them were negative for porcine parvovirus antigen antibodies.
[0053] 3. Experimental method
[0054] 3.1 Single-dose vaccination safety experiment
[0055] 3.1.1 Safety test of piglets
[0056] 20 weaned piglets aged 3 to 5 weeks, 5 piglets in each batch of vaccine, intramuscular injection in the neck, 2mL / head, and the remaining 5 piglets as a control, inoculated with normal saline, carefully observed the reaction of the pigs after injection, and obser...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com