Synthesis method of tetradec-2-yn-1-ol
A synthesis method, fourteen-carbon technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of high complexity of operation, harsh reaction conditions, difficult scale-up production, etc., to achieve purity and yield High efficiency, few reaction steps and short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
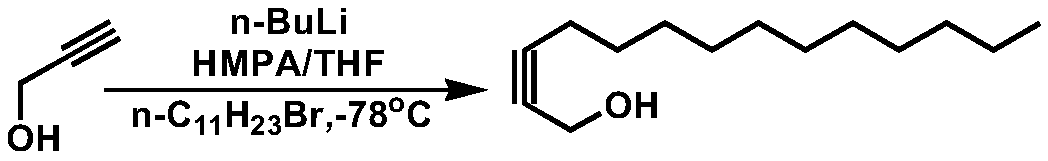
[0031] Add 40g of powdered sodium hydroxide and 600mL of tetrahydrofuran into the reaction bottle, and slowly introduce dry acetylene gas at about 15°C under stirring, and stop the acetylene gas for about 1 hour; control the temperature below 30°C, and add 50g of 1-bromo Undecane / 100mL tetrahydrofuran solution, after the dropwise addition, continue to stir for 5h.
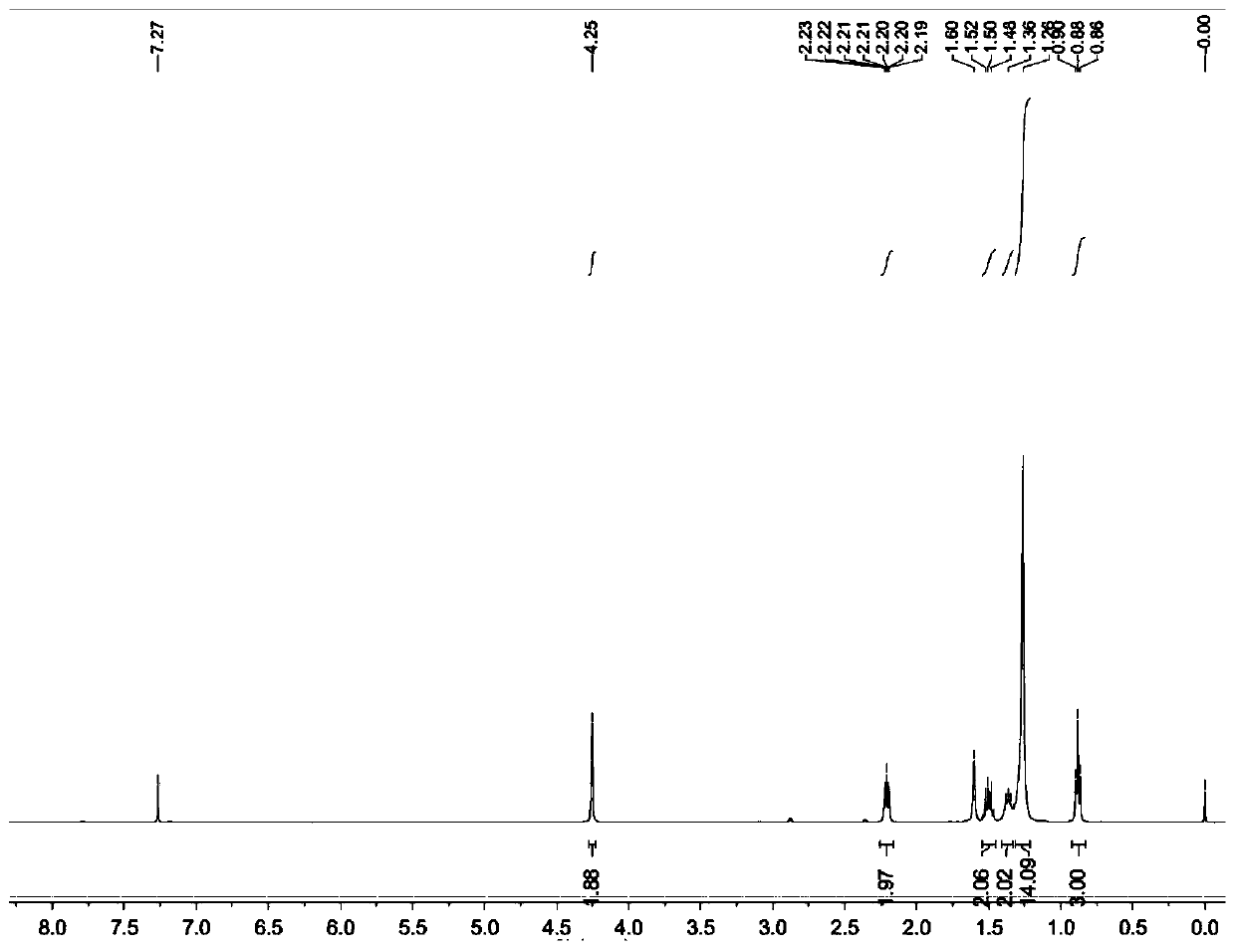
[0032] Add 30g of dry paraformaldehyde, heat to 60°C and react for 2h; concentrate to remove the solvent, add 250mL of water, extract with dichloromethane for 3 times, combine the organic phases, dry, concentrate, and purify the residue by column chromatography (eluent is petroleum ether : ethyl acetate=10: 1), obtain white solid 36g, purity 99.1%, fusing point: 42-43 ℃; Product 2-tetradecyn-1-alcohol nuclear magnetic detection spectrum result is as follows figure 1 shown. Depend on figure 1 It can be seen that 1 H-NMR (400MHz, CDCl 3 )δ0.88(t, J=8Hz, 3H), 1.20-1.36(m, 16H), 1.48-1.52(m, 2H), 1.60(br, 1H), 2.19...
Embodiment 2
[0034] Add 66g of powdered potassium hydroxide (85%) and 800mL of methyl tert-butyl ether into the reaction flask, slowly introduce dry acetylene gas at about 15°C under stirring, and stop the introduction of acetylene gas after the system is saturated for about 1 hour; Below ℃, add 50g 1-bromoundecane / 200mL methyl tert-butyl ether solution dropwise, and continue stirring for 5h after the dropwise addition.
[0035] Add 50 g of dry paraformaldehyde, heat and reflux for 2 h; cool, add 300 mL of water, stir, separate liquids, dry the organic phase, concentrate, and purify the residue by column chromatography (eluent: petroleum ether: ethyl acetate = 10:1 ) to obtain 28g of white solid with a purity of 98.6% and a melting point of 42-44°C.
Embodiment 3
[0037] Add 1L of 1.6mol / L isopropylmagnesium bromide / tetrahydrofuran solution into the reaction flask, cool to 0°C, slowly introduce 20g of dry acetylene gas, and stir for 1h; control the temperature below 20°C, and add 85g of 1-bromo Undecane / 400mL tetrahydrofuran solution, after the dropwise addition, continue to stir for 5h.
[0038] Control the temperature below 10°C, heat 50g of dry paraformaldehyde to 220°C for decomposition, and pass the formaldehyde gas generated by the decomposition into the above reaction solution; heat to reflux for 30min, cool, concentrate to remove the solvent, add 500mL of 10% hydrochloric acid, and extract with dichloromethane Three times, the organic phases were combined, dried, concentrated, and the residue was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10:1) to obtain 68 g of a white solid with a purity of 99.5% and a melting point of 43-44°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com