SUMO fluorescent probe and preparation method thereof
A fluorescent probe, fluorescein technology, applied in the field of protein synthesis, to achieve the effects of high reaction conversion efficiency, low cost and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
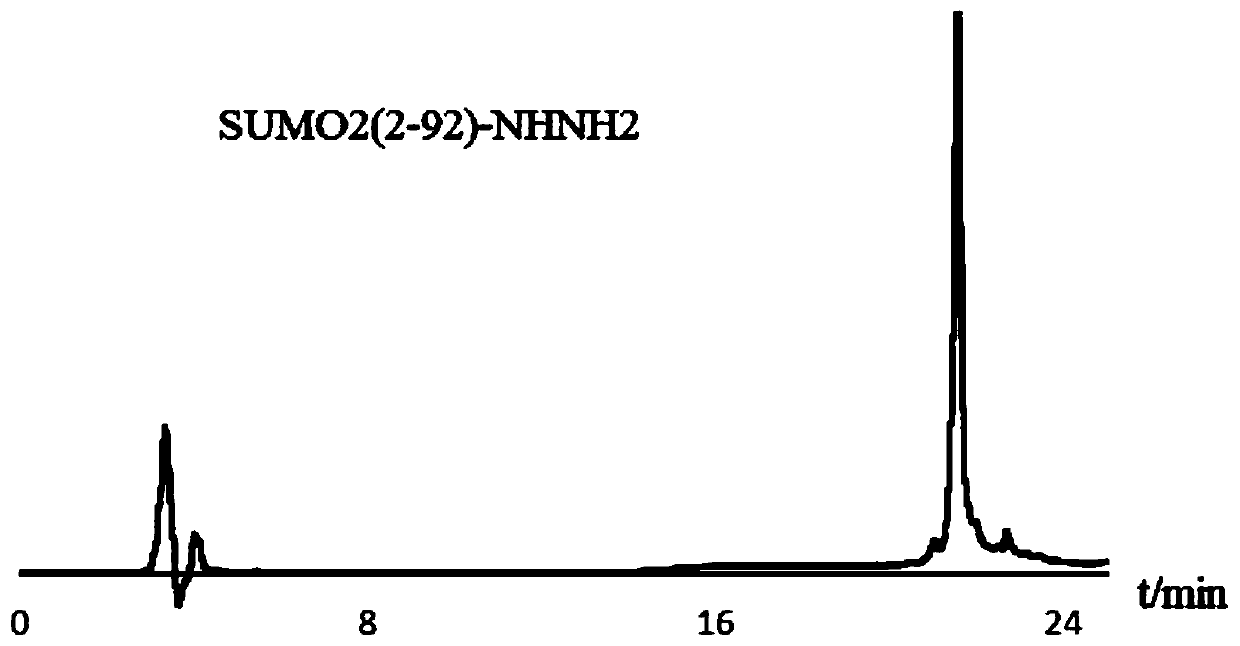
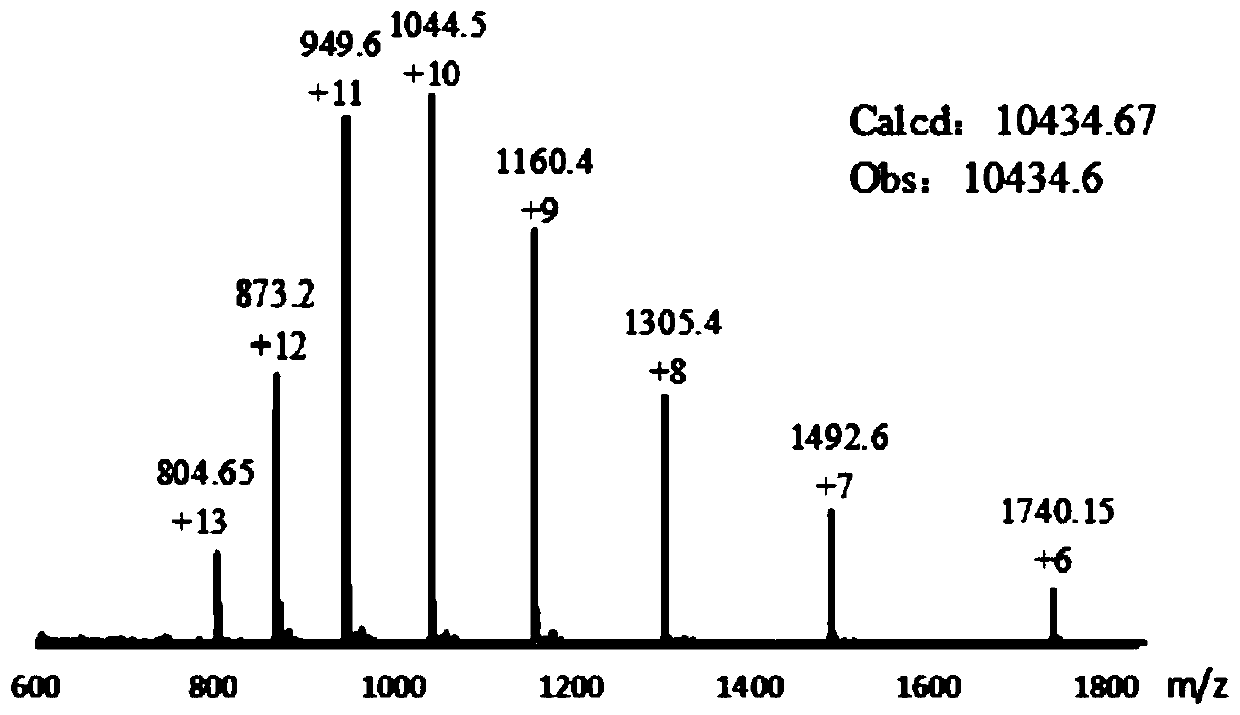
[0057] Example 1 Preparation of SUMO Hydrazide SUMO2(2-92)-NHNH Using the Intein Method N-S Migration Strategy 2 . It is based on commercial Intein-containing strains, and with the help of vectors based on conventional techniques, connect SUMO2 protein to the N-terminus of Intein, connect chitin-binding domain to its C-terminus, and transform Escherichia coli to obtain strains capable of expressing Intein and SUMO2 proteins . Among them, the strain containing Intein is commercialized BL21, purchased from Quanshijin Company, the vector cloned in this strain is PTXB1, and the restriction sites are NdeI and SapI.
[0058] The strain capable of expressing Intein and SUMO2 is cultured, lysed to obtain a supernatant, and then the chitin beads are used for affinity chromatography, and the chitin beads bind to the chitin-binding protein in the supernatant, and the chitin The binding protein is connected to the intein, and the other end of the intein is connected to the SUMO2 target ...
Embodiment 2
[0070] Example 2 Preparation of SUMO thioester SUMO2(2-92)-Mesna by protein hydrazide method, including the following steps:
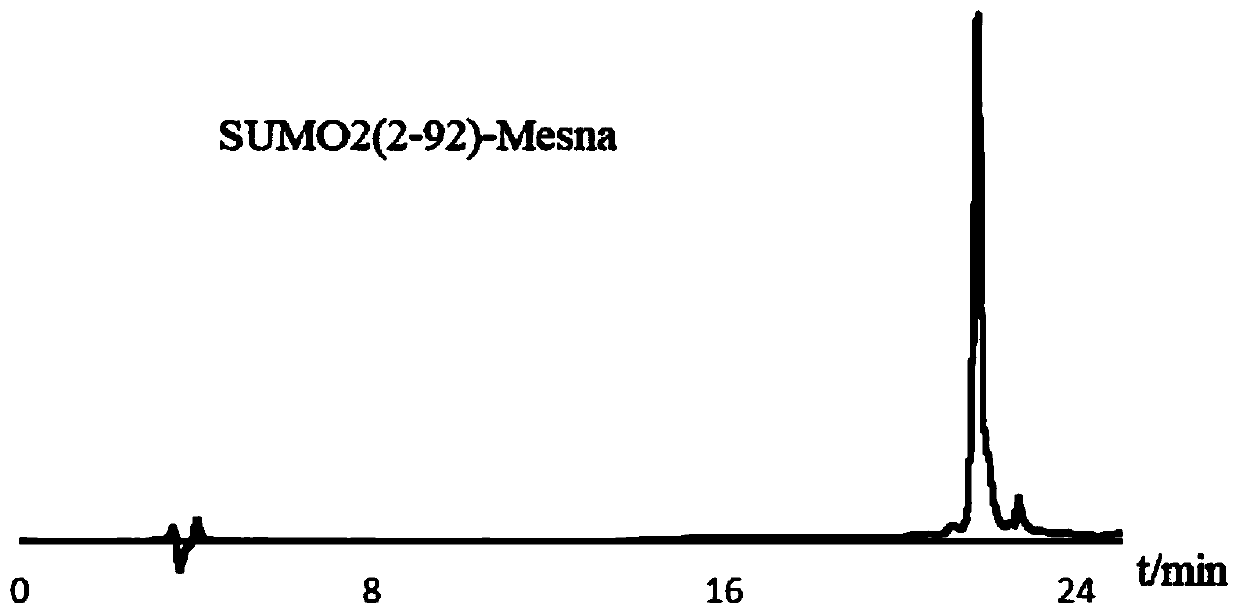
[0071] 50mg of SUMO2(2-92)-NHNH 2 Dissolve in 6mL of 6M guanidine hydrochloride and 0.2M disodium hydrogen phosphate PBS buffer solution with pH=3.0, add 60uL of 1.0M sodium nitrite aqueous solution, and react at -20°C for 20min; after the reaction, add 39mg of Sodium, and adjust the pH to 5.0, and react at room temperature for 20 minutes; use semi-preparative high-performance liquid chromatography after the reaction is completed, such as image 3 As shown, the above reaction solution was purified, the purified solution was collected, and about 6-8 mg of SUMO thioester SUMO2(2-92)-Mesna could be obtained per 1 L of LB medium after lyophilization.
[0072] image 3 It is the high performance liquid chromatogram of SUMO thioester SUMO2(2-92)-Mesna, the abscissa represents the time, and the ordinate represents the absorption value. At the same time, th...
Embodiment 3
[0074] Embodiment 3 provides a kind of method utilizing SUMO thioester SUMO2(2-92)-Mesna to generate SUMO probe SUMO2-Rho110-Gly, which comprises: first by Boc 2 O acid anhydride protects SUMO thioester SUMO2(2-92)-Mesna to form Boc-SUMO2(2-92)-Mesna, followed by direct aminolysis of Boc-SUMO2(2-92)-Mesna to obtain SUMO probe SUMO2-Rho110 -Gly.
[0075] Specifically include the following steps:
[0076] 3a. Weigh 43 mg of SUMO2(2-92)-Mesna protein dry powder, dissolve it in 1.5 mL DMSO (dimethyl sulfoxide), and add 18.5 μL of Boc 2 O acid anhydride (di-tert-butyl dicarbonate) and 8 μL of DIEA (N,N-diisopropylethylamine), stirred at room temperature for 1 h, precipitated the protein with glacial ether, and dried naturally to obtain Boc-SUMO2(2- 92) - Mesna;
[0077] 3b. Dissolve the precipitated protein in 3a in 2.5mL DMSO, add 10μL of thiophenol, and then add 11.5mg of Gly-Rho110-Gly. After the small molecule Gly-Rho110-Gly is completely dissolved, add 8.5μL DIEA, after re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com