A kind of soluble human ige receptor protein truncated protein and its preparation method and application
A technology of receptor protein and truncated protein, which is applied in the field of bioengineering, can solve the problems of different natural proteins, no public expression level, and unidentified activity of proteins, so as to increase expression level, resist protease hydrolysis, correct folding, and improve protein solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
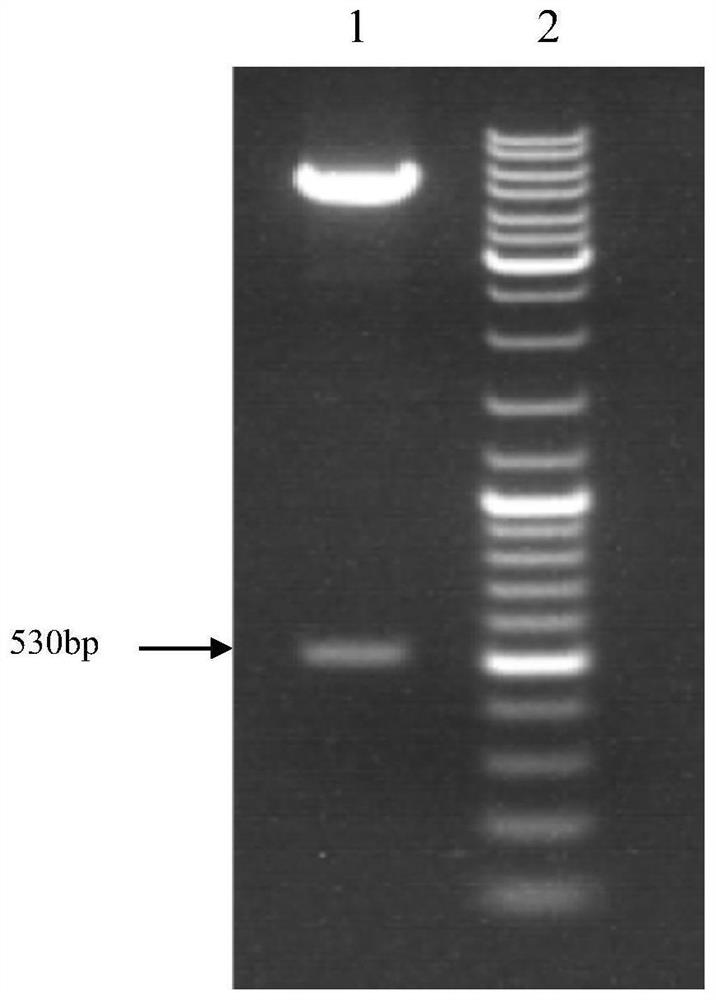
[0033] Embodiment 1: Construction of Escherichia coli expression strain
[0034] The plasmid PET SUMO and Rosetta gami 2 competent cells containing the target gene shown in SEQ ID NO: 2 (synthesized by Guangzhou Aiji Biotechnology Co., Ltd.) were thawed on ice. After complete thawing, mix the two thoroughly in the ultra-clean workbench (50-100ul (10ng / ul) for plasmids, 100-200ul for competent cells, place on ice for 30min, heat shock at 42 degrees for 90s, quickly place on ice for 2 ~5min, add 800~1000μL SOC medium, 37℃, 180rpm culture for 1h, 5000rpm, centrifuge 5~10min, remove most of the supernatant, leave about 50~100μL medium to resuspend the bacteria, evenly spread on ( In AMP+) LB medium, cultivate overnight at 37°C. Pick positive clones with normal colony morphology as bacterial species. After expanding the culture with LB medium, take a small amount of culture fluid and send it to Sangon Bioengineering Shanghai (Stock) Co., Ltd. Extraction and sequencing of the plasm...
Embodiment 2
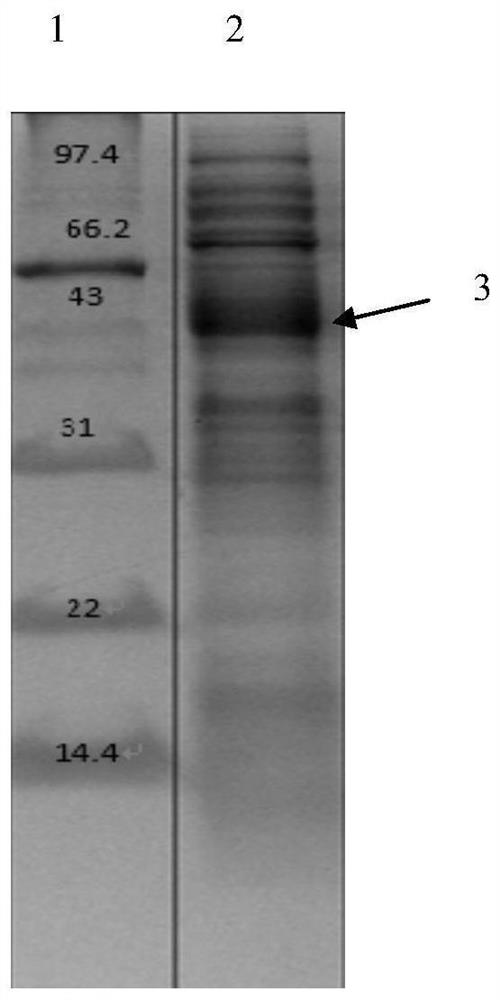
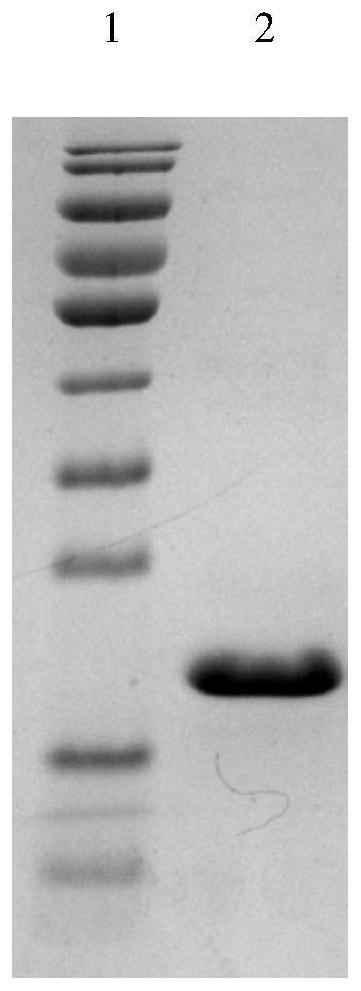
[0035] Example 2: Expression and purification of receptor protein truncated protein
[0036] Prepare sterilized LB liquid medium (add 2g / L glucose), add kanamycin (final concentration is 100μg / ml), pick a single colony with the tip of a pipette, culture at 37°C, 180rpm, every Measure OD every 1 hour 600 , record the data, and add the inducer IPTG (final concentration is 1mol / L) in the rapid growth period, continue to cultivate, and measure the OD every 1h 600 , record data, finally get its growth curve, measure OD 600 When it was observed that the absorbance value did not change significantly, the cultivation was stopped, and the cells were collected by centrifugation at 5000 rpm for 10 minutes. In an ice bath, 1 g of bacteria was added to 5 mL of PBS solution, the power of ultrasonic crushing was 300 W, and the crushing time was 4 seconds and 4 seconds, the total time was 5 minutes, and then centrifuged at 8000 rpm for 5 minutes to collect the crushed supernatant. The nick...
Embodiment 3
[0037] Example 3: Synthesis of IgE Immunosorbent
[0038] Take 5mL of agarose GE Sepharose 6FF, add 8mL of 2M sodium hydroxide and 5mL of bisglycidyl ether, 40°C, 180rpm, 5h, after the reaction, filter the filler, and wash the filler with about 100mL of purified water until neutral. The activated agarose was added to 5 mL of receptor protein solution with a concentration of 15 mg / mL, and reacted at 20° C. and 180 rpm for 15 h. After the reaction is completed, use about 100ml of purified water to pump and wash the packing until neutral. Finally, 10 mL of 1M ethanolamine (pH=8.0) solution was added and mixed, and reacted at 20°C and 180 rpm for 10 h. Wash the filler with about 100ml of purified water to obtain the IgE immunosorbent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com