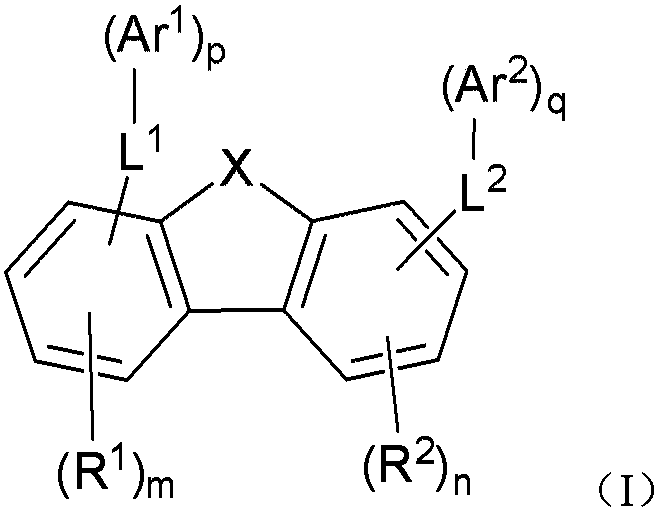
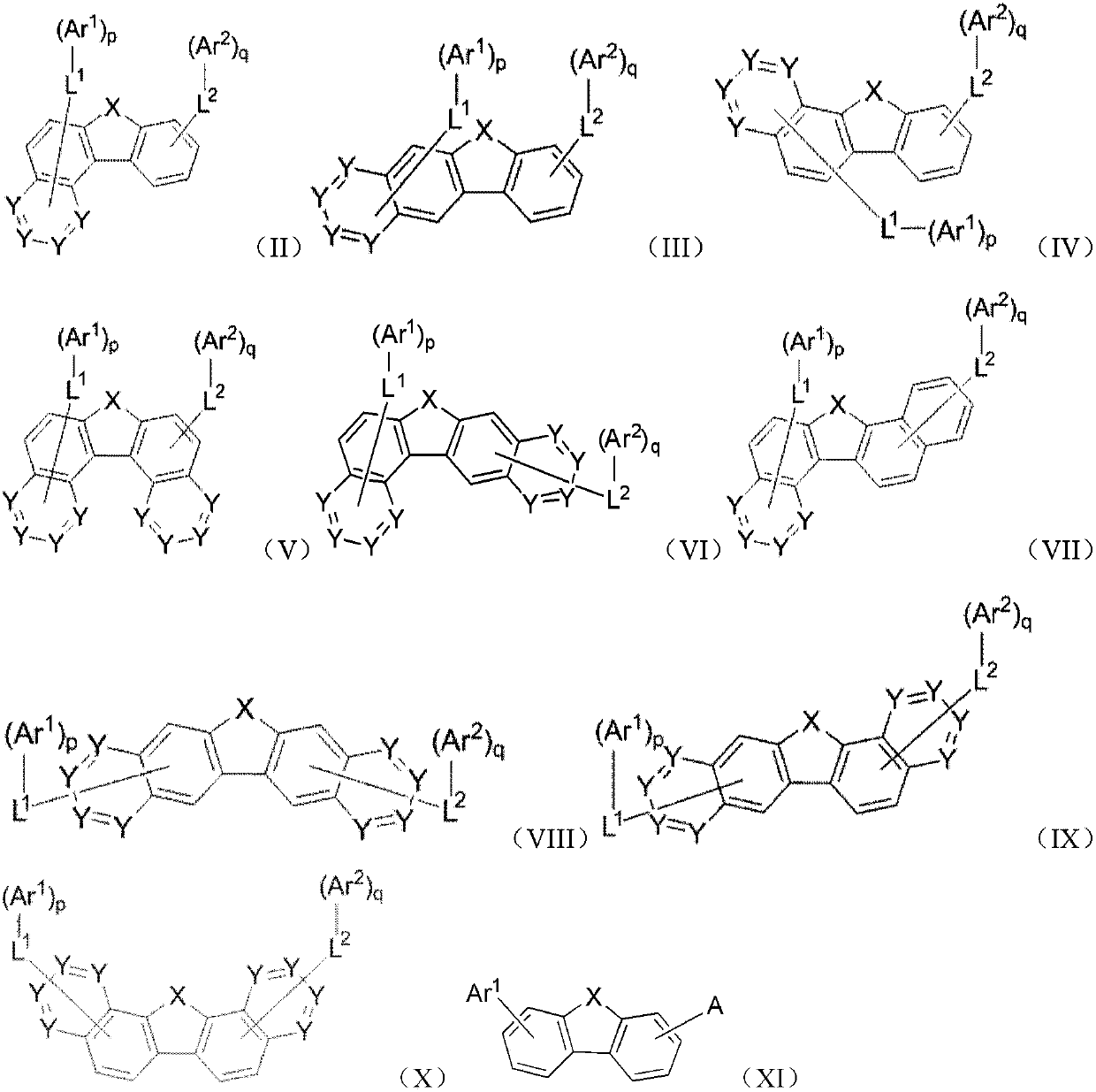
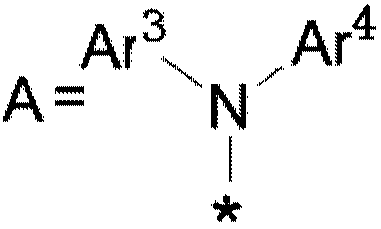
Organic compound and application of organic compound in organic electroluminescent device
A technology of organic compounds and compounds, applied in organic chemistry, electrical solid-state devices, electrical components, etc., can solve problems such as mismatch between electrons and holes in the light-emitting layer, shortened lifespan, and efficiency roll-off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0069] Synthesis Example 1: Synthesis of Compound A1
[0070]
[0071] In the reaction flask, add 21.8g (100mmol) of 2-methylthio-1-naphthaleneboronic acid, 16.5g (110mmol) of bromobenzene, 0.9g (0.785mmol, 0.5%) of tetrakis(triphenylphosphine palladium), 1500ml toluene, ethanol 1000ml, potassium carbonate 43.3g (314 mmol) / water 1000ml, react at 80°C for 3.5h. After the reaction is complete, stop the reaction. After cooling to room temperature and filtering, the obtained solid was purified by recrystallization from toluene to obtain white powder M1.
[0072] Intermediate M132.4g (100mmol) was dissolved in 150ml of glacial acetic acid, 30% hydrogen peroxide (5ml) was added in ice bath at 0°C, reacted at room temperature for 10h, after stopping the reaction, extracted, dried, evaporated the solvent, and the column layer Separated by analysis, a white solid M2 was obtained.
[0073] Intermediate M2 26.9g (100mmol) and phosphorus pentoxide (10mmol) were added to 20ml of tr...
Synthetic example 2
[0078] Synthesis of Synthesis Example 2 Compound A8
[0079]
[0080] In the reaction flask, add 25.2g (100mmol) of 1-methylthio-2-naphthaleneboronic acid-7-chloro, 30.6g (110mmol) of m-bromoiodobenzene, 0.9g (0.785mmol, 0.5% ), toluene 1500ml, ethanol 1000ml, potassium carbonate 43.3g (314mmol) / water 1000ml, react at 80°C for 3.5h. After the reaction is complete, stop the reaction. After cooling to room temperature and filtering, the obtained solid was purified by recrystallization from toluene to obtain white powder M1.
[0081] 36.3g (100mmol) of intermediate M1 was dissolved in 150ml of glacial acetic acid, 30% hydrogen peroxide (5ml) was added in ice bath at 0°C, and reacted at room temperature for 10h. After stopping the reaction, extracted, dried, evaporated the solvent, and the column layer Separated by analysis, a white solid M2 was obtained.
[0082] Intermediate M2 37.9g (100mmol) and phosphorus pentoxide (10mmol) were added to 20ml of trifluoromethanesulfon...
Synthetic example 3
[0085] Synthesis Example 3: Synthesis of Compound A19
[0086]
[0087] In the reaction flask, add 21.8g (100mmol) of 2-methylthio-1-naphthaleneboronic acid, 16.5g (110mmol) of bromobenzene, 0.9g (0.785mmol, 0.5%) of tetrakis(triphenylphosphine palladium), 1500ml toluene, ethanol 1000ml, potassium carbonate 43.3g (314 mmol) / water 1000ml, react at 80°C for 3.5h. After the reaction is complete, stop the reaction. After cooling to room temperature and filtering, the obtained solid was purified by recrystallization from toluene to obtain white powder M1.
[0088] 32.4g (100mmol) of intermediate M1 was dissolved in 150ml of glacial acetic acid, and 30% hydrogen peroxide (5ml) was added in an ice bath at 0°C, and reacted at room temperature for 10h. After the reaction was stopped, extraction, drying, evaporation of solvent, and column layer Separated by analysis, a white solid M2 was obtained.
[0089] Intermediate M2 26.9g (100mmol) and phosphorus pentoxide (10mmol) were ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com