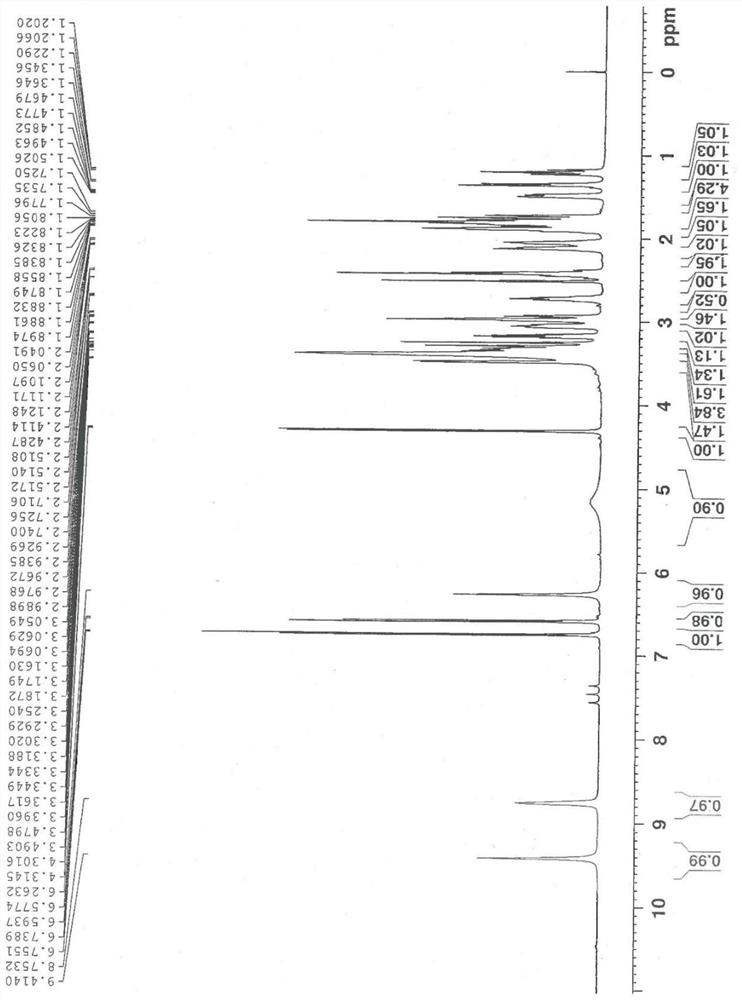
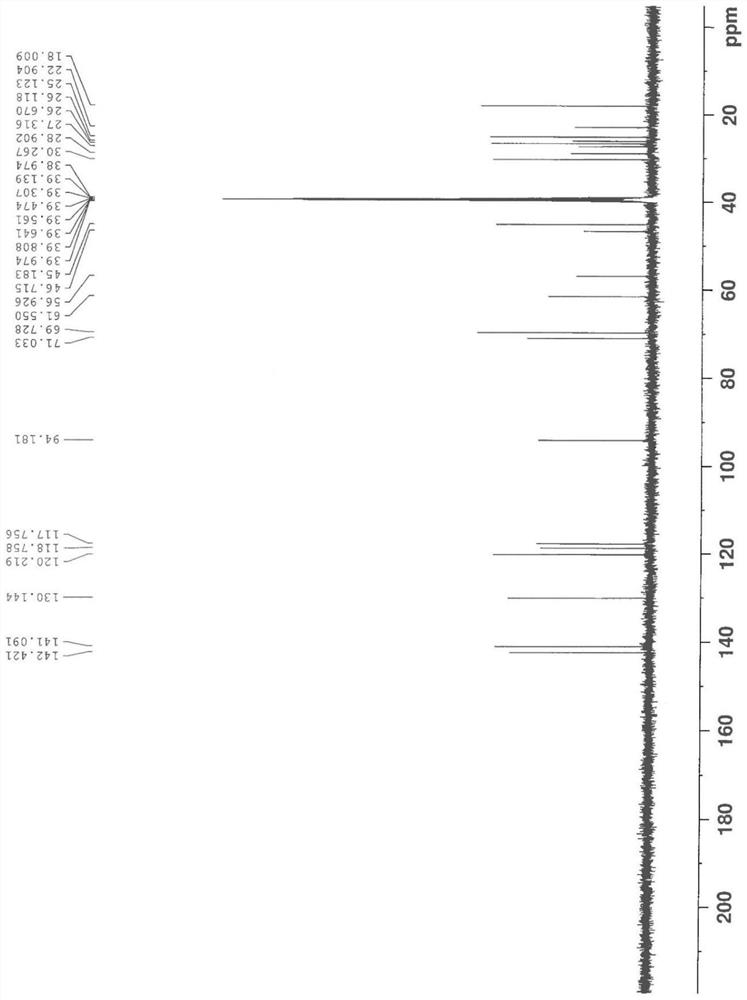
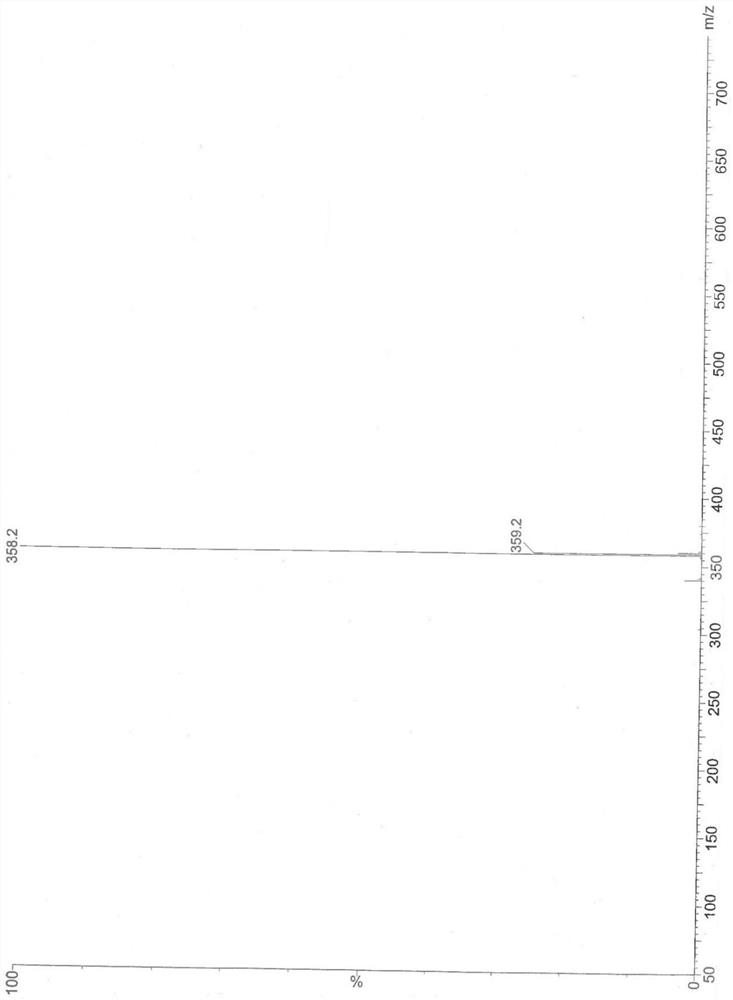
A Stereoselective Synthesis Method of 6β-Hydroxy-7,8-Dihydro-morphine Derivatives
A synthesis method and derivative technology, applied in organic chemistry and other directions, can solve problems such as unreported synthesis methods, and achieve the effects of improving accurate positioning and characterization, cheap raw materials, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The synthesis of embodiment 1β-nalbuphine isomer (formula I-1 compound)
[0108] S1: Add 300ml of chloroform, 28.5g of morphine, and 42.7g of potassium bicarbonate into a 500ml three-necked bottle, stir at room temperature, add 85.5ml of ethyl chloroformate dropwise, after the addition is complete, heat up to reflux until the reaction is complete, cool down to room temperature, and react Pour the solution into 500ml of water, separate the layers, extract the aqueous layer with 300ml of dichloromethane, combine the organic phases, wash with 300ml of water and 200ml of saturated aqueous sodium chloride solution, dry over anhydrous magnesium sulfate, filter, and concentrate under reduced pressure to obtain the residue Thing 43g, promptly obtains formula II compound (R 1 and R 3 Both are ethoxycarbonyl), this intermediate does not need to be purified, it is directly used in the next step with a yield of more than 100%, which may contain ethyl chloroformate.
[0109] S2: A...
Embodiment 2
[0122] S1: Add 300ml of chloroform, 28.5g of morphine, and 42.7g of sodium bicarbonate into a 500ml three-necked flask, stir at room temperature, add 85.5ml of methyl chloroformate dropwise, after the addition is complete, heat up to reflux until the reaction is complete, cool down to room temperature, and react Pour the solution into 500ml of water, separate the layers, extract the aqueous layer with 300ml of dichloromethane, combine the organic phases, wash with 300ml of water and 200ml of saturated aqueous sodium chloride solution, dry over anhydrous magnesium sulfate, filter, and concentrate under reduced pressure to obtain the residue Thing 42g, promptly obtains formula II compound (R 1 and R 3 Both are methoxycarbonyl), this intermediate is directly used in the next step without purification, and the yield is over 100%, which may contain methyl chloroformate.
[0123] S2: Add acetone 500ml, S1 formula II compound (R 1 and R 3 Both are methoxycarbonyl), stir to dissolv...
Embodiment 3
[0132] Preparation of β-6-hydroxyhydromorphone (as shown in formula (I-2))
[0133] S1: Add 28.5g of hydromorphone, 300ml of acetonitrile, and 10.2g of triethylamine into a 500ml three-necked bottle, cool down to 0°C, add 10.2g of acetic anhydride dropwise, after the dropwise addition, return to room temperature naturally, and the reaction is completed. The solution was poured into 300ml of ice water, extracted twice with 500ml of dichloromethane, washed with saturated aqueous sodium bicarbonate until neutral, washed with saturated aqueous sodium chloride, dried overnight with anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain 330g of white Solid (Intermediate VI-2, R 1 is acetyl, R 3 For methyl), this solid may contain part of the solvent, but it does not affect the next step reaction and can be directly used in the next step reaction.
[0134] S2: Add the compound of formula VI-2 (R 1 is acetyl, R 3 methyl), 560ml of anhydrous methanol, 1.5ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com