Application of bifidobacterium adolescentis CCFM1061 in preparation of functional microbial agents, foods and/or medicines
A functional bacterial agent, bifidobacteria technology, applied in bifidobacteria, bacteria used in food preparation, applications, etc., can solve problems such as liver function damage and edema, increased drug toxicity and side effects, and adverse reactions in the digestive tract. To achieve the effect of improving the increase of total cholesterol, strong adsorption capacity and alleviating the toxicity of PFOA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Bifidobacterium adolescentis CCFM1061 has good tolerance to simulated gastrointestinal fluid
[0061] Inoculate cryopreserved Bifidobacterium adolescentis CCFM1061 in mMRS medium (MRS medium + 0.05% cysteine hydrochloride), culture anaerobically at 37°C for 48 hours, and subculture in mMRS medium for 2-3 After the second time, take 1 mL of the culture solution of Bifidobacterium adolescentis CCFM1061, mix it with 9.0 mL of pH 2.5 artificial simulated gastric juice (mMRS medium containing 1% pepsin, pH = 2.5), and culture it anaerobically at 37°C, respectively. Samples were taken at 0h, 0.5h, 1h, and 2h, and the mMRS agar medium was poured to culture the plate colonies, and the number of viable bacteria was determined and the survival rate was calculated.
[0062] The survival rate is the ratio of the logarithmic value of the number of viable bacteria at the time of sampling to the logarithmic value of the number of viable bacteria at the 0th hour in the cul...
Embodiment 2
[0068] Example 2: Bifidobacterium adolescentis CCFM1061 has no toxic side effects on C57BL / 6J mice
[0069] The Bifidobacterium adolescentis CCFM1061 bacteria were resuspended in 3% sucrose solution to make a concentration of 3.0×10 9 CFU / mL bacterial suspension. Eight healthy male C57BL / 6J mice with a body weight of about 16-20 g were taken. After one week of adaptation to the environment, the bacterial suspension of this concentration was administered orally once a day, observed for one week, and the death and body weight were recorded.
[0070] The results of these tests are listed in Table 3. These results indicate that feeding concentrations of 3.0×10 9 CFU / mL of Bifidobacterium adolescentis CCFM1061 had no significant effect on mice, no significant change in body weight, and no death. The appearance of the mice had no obvious pathological symptoms.
[0071] Table 3 Changes in body weight and death of mice
[0072]
[0073] Note: -: no death of mice
Embodiment 3
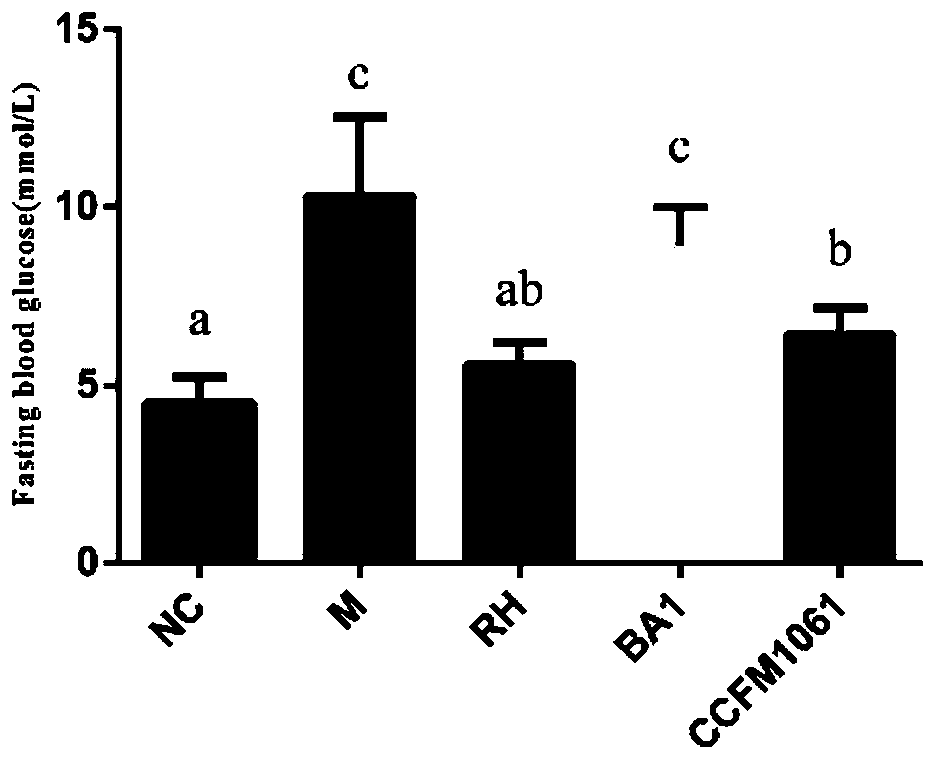
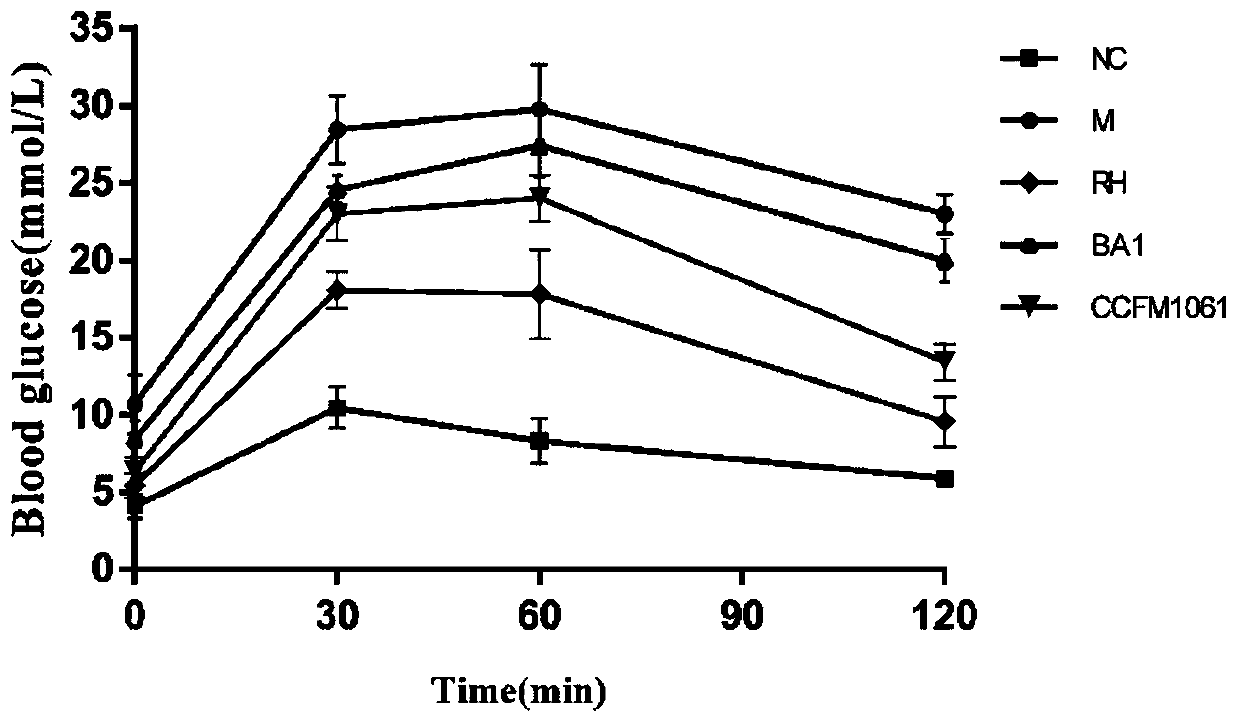
[0074] Embodiment 3: Bifidobacterium adolescentis CCFM1061 can reduce (fasting) blood sugar level in type 2 diabetic mice
[0075] Take 40 healthy male C57BL / 6J mice weighing 16-20g, adapt to the environment for 1 week, and divide them into 5 groups randomly: blank control group (NC), model control group (M), rosiglitazone control group (RH) , Bifidobacterium adolescentis CCFM1061 intervention group (CCFM1061), Bifidobacterium adolescentis BA1 control group (BA1) each group contained 8 mice, and the dose of intragastric bacterial suspension was 3.0×10 9 CFU / mL, resuspended in 3% sucrose solution. The grouping and treatment methods of experimental animals are shown in Table 4:
[0076] Table 4 Grouping of experimental animals
[0077]
[0078]
[0079] Week 2-7: The mice in the normal group were fed with normal feed, and the rest of the mice were fed with high-fat feed.
[0080] On the 1st day of the 11th week, all mice were fasted for 12 hours, the normal group was in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com