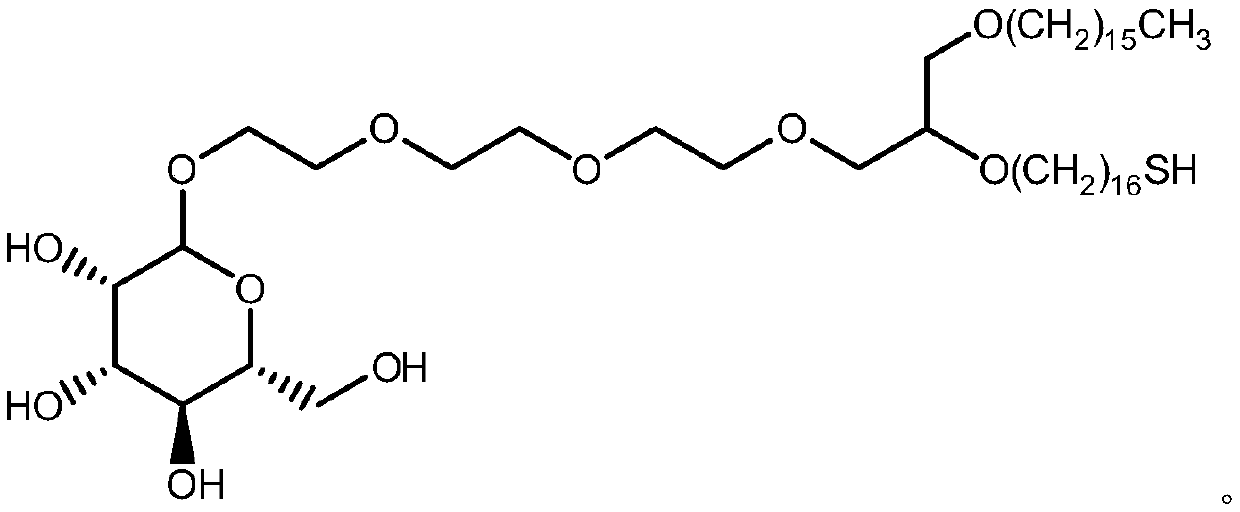
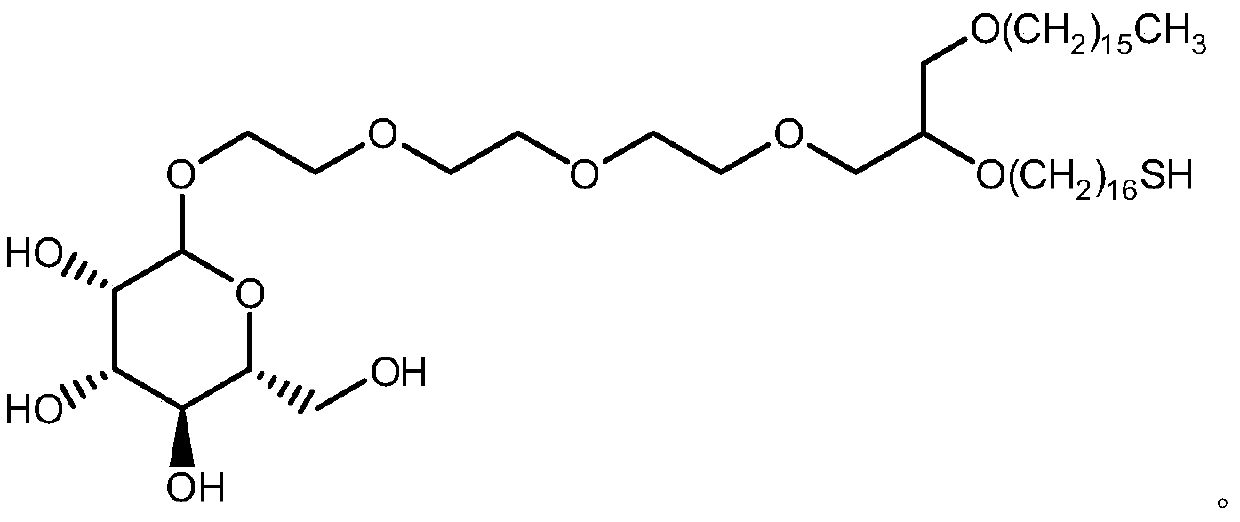
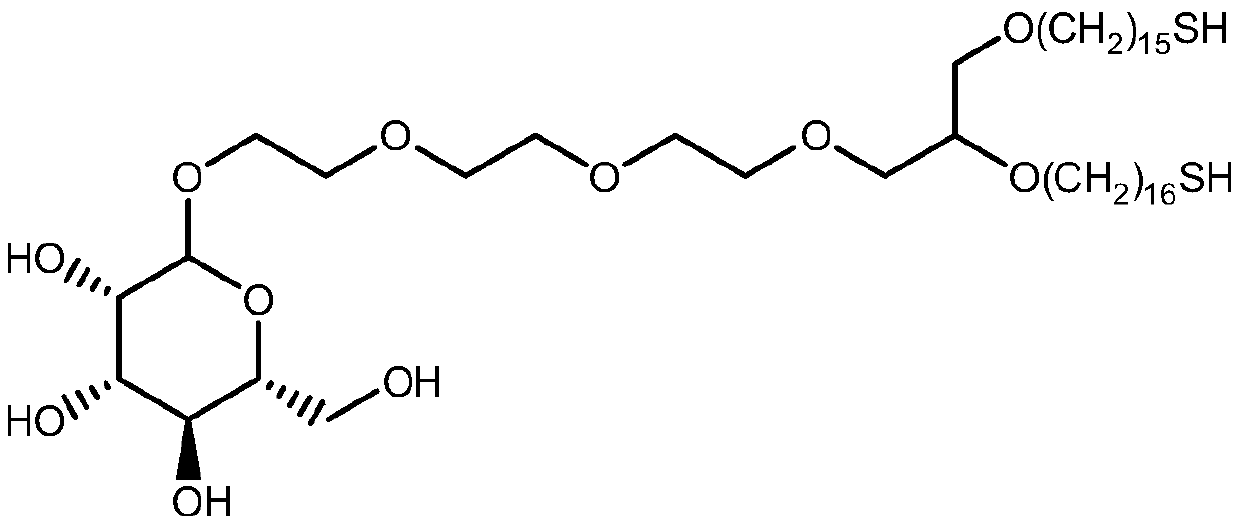
Synthesis method of mono-sulfhydryl bis-hexadecyl ether polyethylene glycol interchain oligosaccharide glycolipid
A technology of monomercapto dihexadecyl ether and triphenylthio dihexadecyl ether, which is applied in the field of chemical synthesis and can solve the problem of difficulty in obtaining amphiphilic aliphatic chain polyethylene glycol interchain glycolipids containing mercapto groups, etc. problem, to achieve the effect of low breakthrough yield, high product yield, simple and efficient method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1) Synthesis of Allyloxytriethylene Glycol (TG-Ally)
[0064] Dissolve 10 mL of triethylene glycol in 30 mL of dry THF, stir at room temperature for 30 min, add 3.5 g of pure sodium and stir rapidly for 1 h, add dropwise 15 mL of allyl bromide, and continue the reaction for 6 h. The residue after vacuum distillation was dissolved in 30 mL of dichloromethane, washed with saturated NaCl solution, dried over anhydrous magnesium sulfate, filtered and concentrated, and purified by column chromatography to obtain 4 g of a colorless oily product with a yield of 80%.
[0065] Column chromatography: Silica gel: Et 2 O / EtOAc 1:1
[0066] (2) Synthesis of allyloxytriglycol acyl mannose
[0067] 0.5g 2,3,4,6-O-acetyl-α-D-mannopyranose trichloroacetimidate and 1.25g TG-Allyl were mixed and dissolved in 10mL dry THF, stirred at room temperature for 30min, quickly Add 125 μL TMSOTf dropwise, after stirring continuously for 30 h, add 125 μL ethanolamine to decompose excess TMSOT, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com