Monooxygenase DszC mutant and preparation method and application thereof
A monooxygenase and mutant technology, applied in the field of genetic engineering, can solve problems such as binding sites and binding mechanisms that have not yet been resolved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, use pSB4A5-BADC plasmid to construct the mutant library of dszC gene
[0034] (1) Design random mutation primers EP-S / EP-A.
[0035] EP-S:5'-ATCTGTTGTTTGTCGGTGAACGCTCTCTAC-3' (SEQ NO:5)
[0036] EP-A: 5'-TTTACAAAAAACCCCTCAAGACCCGT-3' (SEQ NO: 6)
[0037] Using the nucleotide sequence of wild-type DszC shown in Seq1 as a template, DszC was randomly mutated by using error-prone PCR primers EP-S / EP-A, using low-fidelity DNA amplicon rTaq enzyme, and adding MnSO4. The mutant gene of dszC and the expression vector pSB4A5-BAD containing the other three genes dszA, dszB and dszD of the 4S pathway were digested with Nhe I and Hind III and ligated with T4 ligase to transform the cloning host E.coli BL21 (DE 3 ). The obtained mutants were picked one by one into a sterile 96-well plate, and cultured overnight in LB medium containing ampicillin with shaking at 35-38°C to obtain the mother plate. Copy the bacteria in the master plate to a deep-well 96-well plate to...
Embodiment 2
[0038] Embodiment 2, the re-screening of the mutant shake flask fermentation obtained by orifice screening
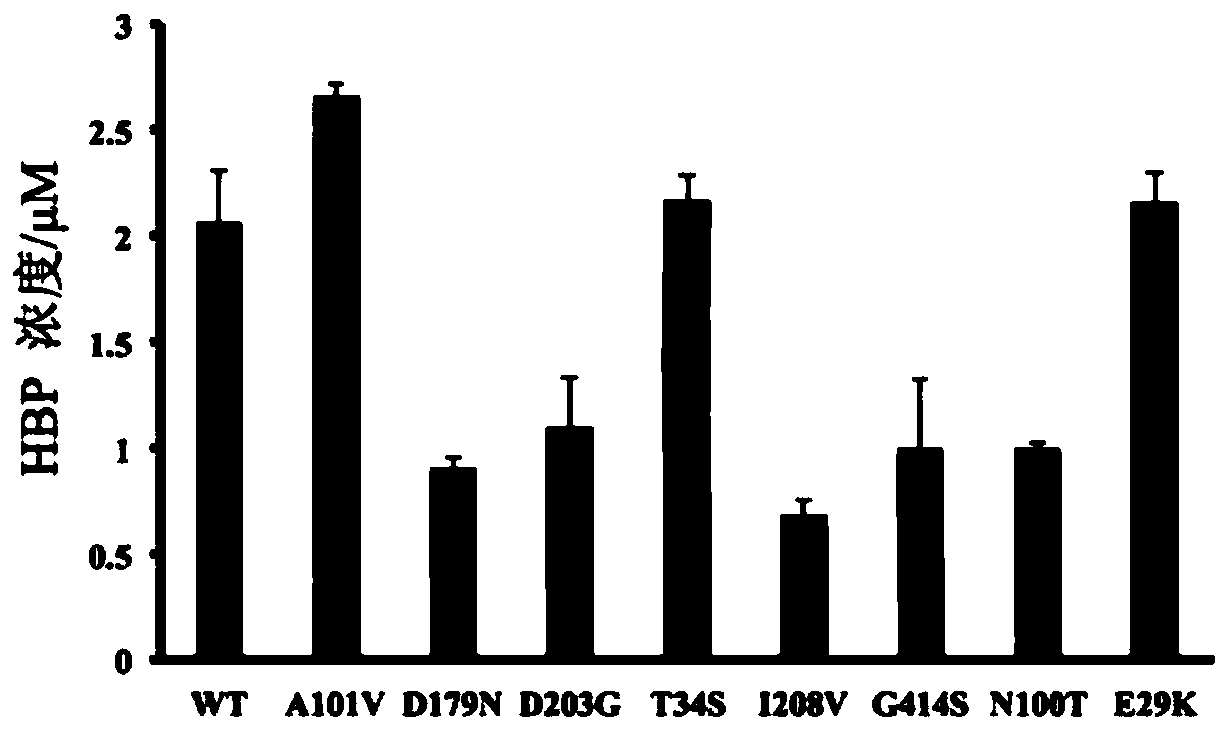
[0039] Pick A from Motherboard 610 / A 600The strains with higher yield than wild-type HBP were sequenced, and the strains with amino acid changes were selected for re-screening in shake flasks. The cells were collected and washed after induced fermentation, and a certain amount of cells and a certain amount of DBT were controlled in a 5mL reaction system as whole-cell catalytic substrates. After shaking and reacting at 27-35°C for 24 hours, take a certain amount of reaction solution, extract it with an equal volume of ethyl acetate, and use HPLC to detect the content of each component in the reaction system. Through high-throughput screening and simulated molecular docking, the mutant strain E.coli BL21(DE3) / A101V with the highest HBP production was obtained, which was 28.81% higher than the strain containing wild-type DszC. The result is as figure 1 shown.
Embodiment 3
[0040] Example 3, iterative saturation mutation of DszC
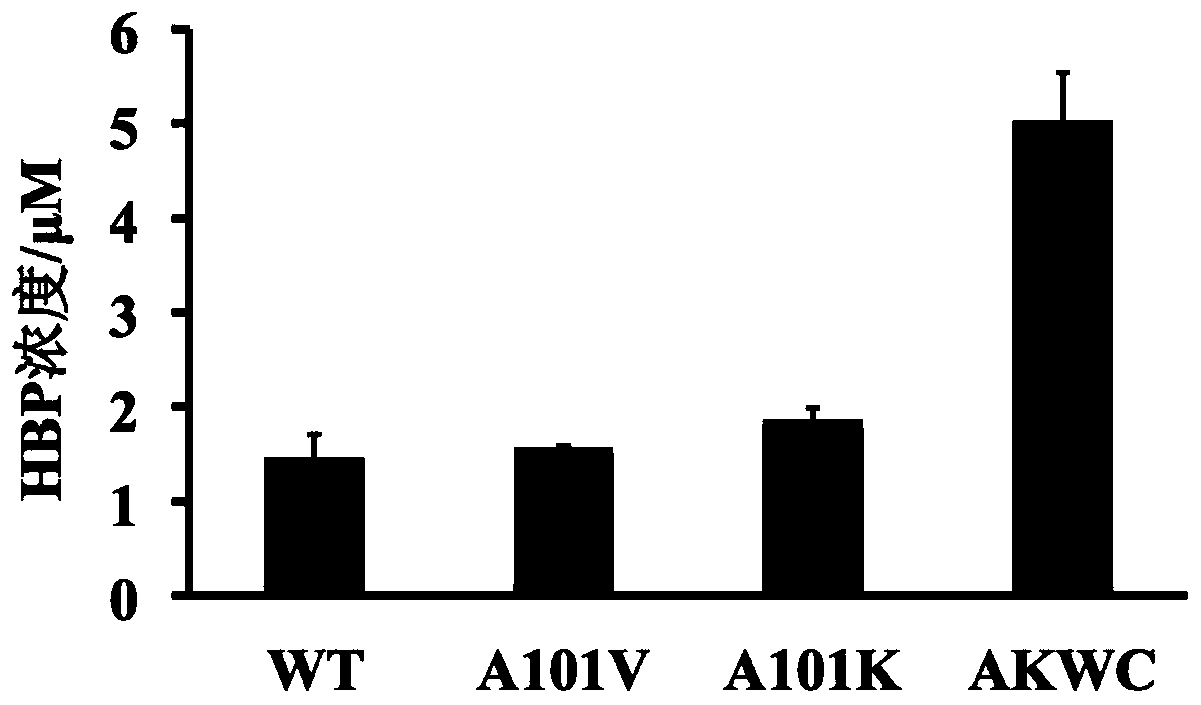
[0041] The wild-type DszC was subjected to saturation mutation of amino acid A101 by site-directed mutagenesis primers, and the obtained mutants were expressed and purified, and their enzymatic activities were measured under the inhibitory effect of a certain concentration of HBP. Among them, A101K has the highest enzyme activity, which is 29.51% higher than that of WT.
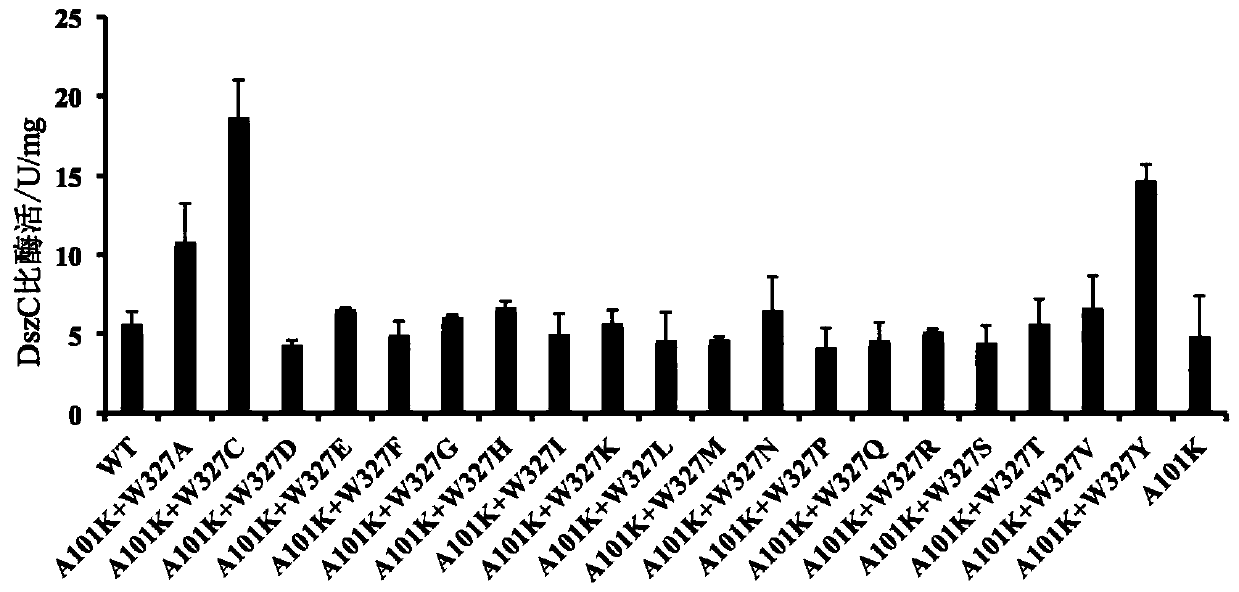
[0042] Using A101K as a template, DszC was subjected to iterative saturation mutation with site-directed mutagenesis primers. After the obtained mutants were expressed and purified, their enzyme activities were measured under the inhibitory effect of a certain concentration of HBP. Such as figure 2 As shown, A101K / W327C (marked as AKWC) has the highest enzyme activity, which is 3.3 times higher than that of WT. The enzymatic activity of purified AKWC was measured under HBP inhibition, and its IC 50 The increase from μM level to mM level indicates ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Desulfurization efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com