Method for obtaining On-DNA aromatic hydrocarbon compound by Suzuki coupling reaction in construction of DNA encoded compound library
A technology for aromatic compounds and compound libraries, applied in the field of DNA-encoded compound libraries, can solve problems such as limited substrate applicability, unstable reagents, and easy deterioration, and achieve the effects of increasing diversity, convenient operation, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, the synthesis of On-DNA aryl bromide
[0050]
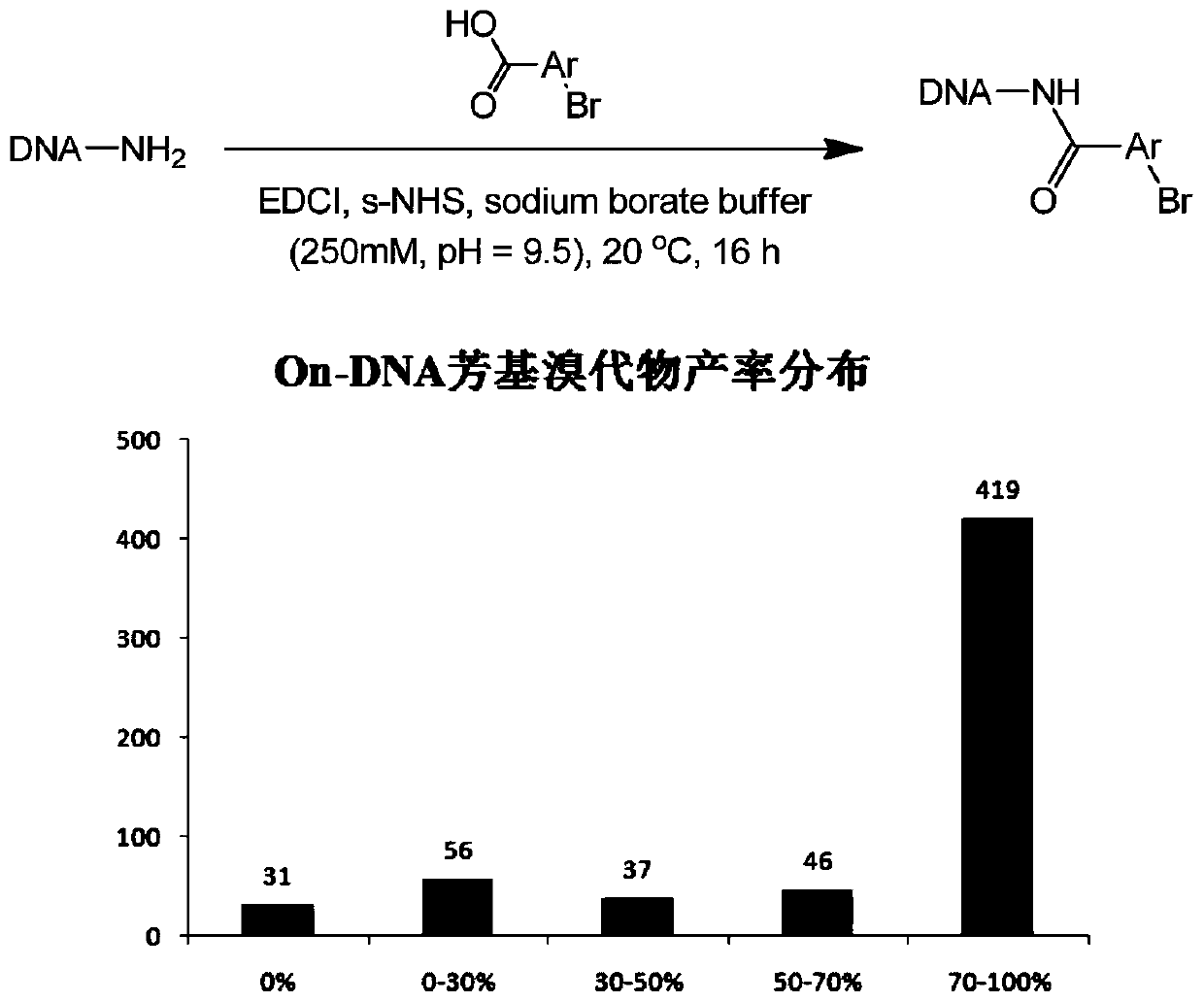
[0051] DNA-NH 2 (For example, the starting head fragment mentioned in the patent CN108070009A) was dissolved in 250mM, pH=9.5 boric acid buffer solution, prepared into a 1mM concentration solution, dispensed into a 96-well plate, and mixed with the bromoaryl carboxyl binary compound (total 569) using EDCI as a condensing agent and s-NHS condensation activator to react to obtain the corresponding On-DNA aryl bromide (abbreviated as DNA-Ar-Br, reference: Nat.Chem., 2015, 7, 3, 241), After this reaction is completed, only ethanol precipitation is done, and after concentration and drying, it is directly used in the reduction reaction of the next step (see figure 1 ).
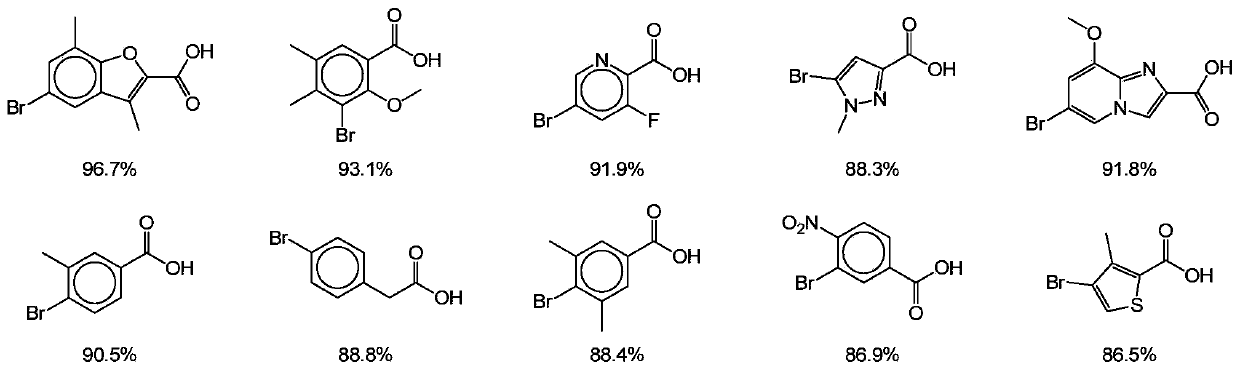
[0052]We have reacted a total of 589 different bromoaryl carboxyl binary compounds, but in order to reduce the impact of the inconsistent yield of the first condensation reaction on the calculation of the yield of the second Suzuki coupling re...
Embodiment 2
[0053] Embodiment 2, the synthesis of On-DNA aryl olefin compound
[0054]
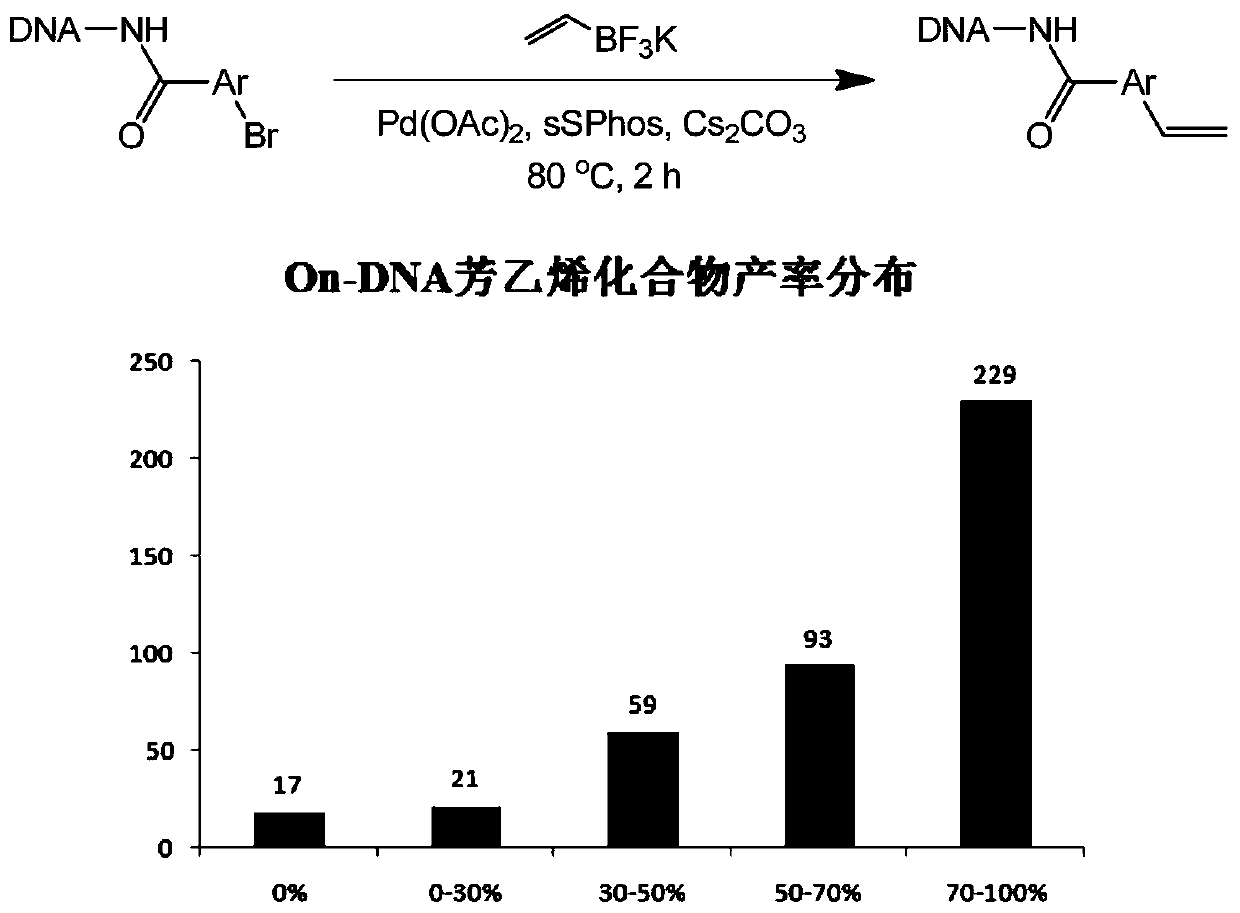
[0055] The DNA-Ar-Br generated in situ was redissolved in ultrapure water, and prepared into a 1mM concentration solution. Two aliquots were taken out and distributed into new 96-well plates (5μL, 5nmol, 1mM aqueous solution), and vinyl Potassium trifluoroborate (1.25μL, 250nmol, 200mM dimethylacetamide solution, 50eq.), cesium carbonate solution (3.75μL, 750nmol, 200mM aqueous solution, 150eq.), centrifuge to allow the solution to sink to the bottom, vortex to mix, and again After centrifugation, nitrogen was replaced 3 times, and palladium acetate (Pd(OAc) 2 ) and a premixed solution (1.5 μL, volume ratio = 2 / 1, 10mM dimethylacetamide solution / 40mM dimethylacetamide solution, 2eq. / 4eq.), replace nitrogen 3 times again, seal the film, and react 2 hours (cover temperature: 100°C).
[0056] Palladium removal: After the reaction is completed, add sodium diethyldithiocarbamate solution (3.0 μL, 300...
Embodiment 3
[0060] Embodiment 3, the synthesis of On-DNA benzylamine compound
[0061]
[0062] DNA-Ar-Br (5 μL, 5 nmol, 1 mM aqueous solution) was added to a 96-well plate, followed by adding N-Boc-aminomethyl potassium trifluoroborate (3.75 μL, 750 nmol, 200 mM dimethylacetamide solution, 150 eq.), Potassium carbonate solution (7.5μL, 1500nmol, 200mM aqueous solution, 300eq.), centrifuged to allow the solution to sink to the bottom, vortexed to mix well, after centrifuging again, replace nitrogen 3 times, add palladium acetate (Pd(OAc) 2 ) and 4-(2,6-dimethoxyphenyl)-3-(1,1-dimethylethyl)-2,3-dihydro-1,3-benzoxaphosphapan Pre-mixed solution of rac-BI-DIME (5.0 μL, volume ratio=1 / 1, 10 mM dimethylacetamide solution / 40 mM dimethylacetamide solution, 5eq. / 20eq.), again Nitrogen was replaced 3 times, the film was sealed, and the 96-well plate was reacted at 95° C. for 2 hours in a PCR instrument (cover temperature: 105° C.).
[0063] After the reaction is complete, add sodium diethyldi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com