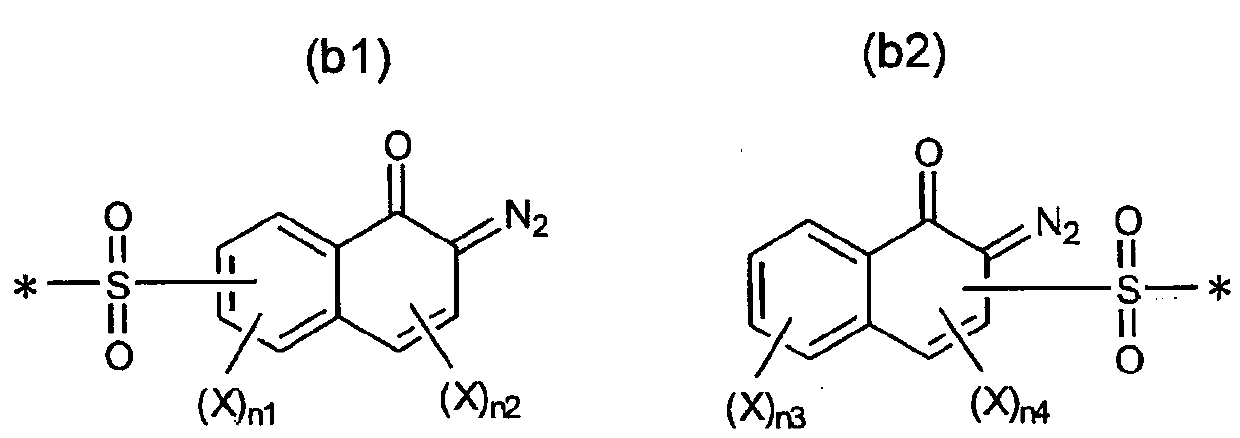
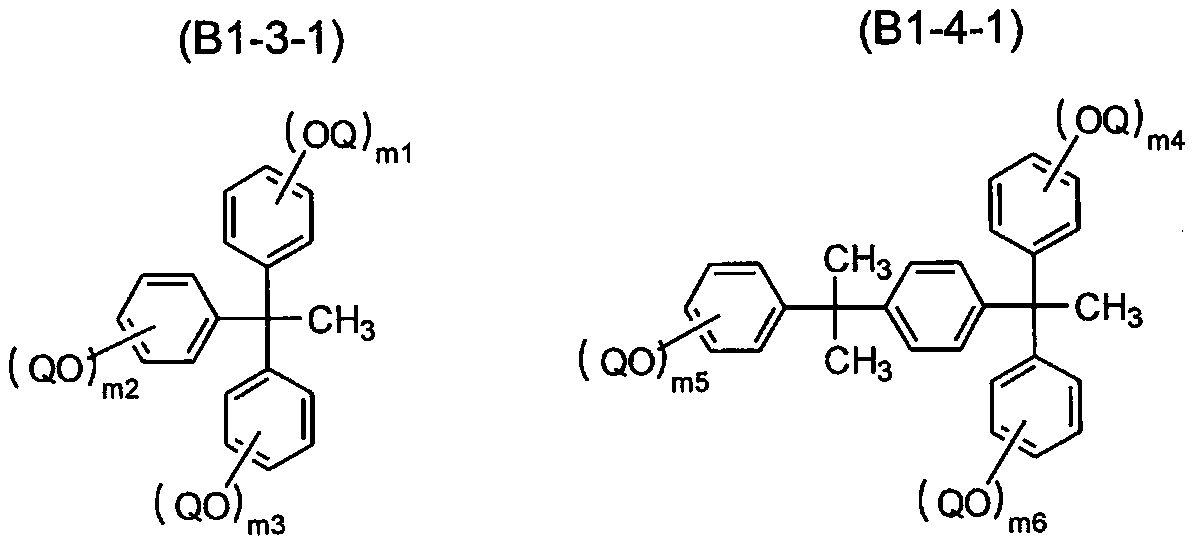
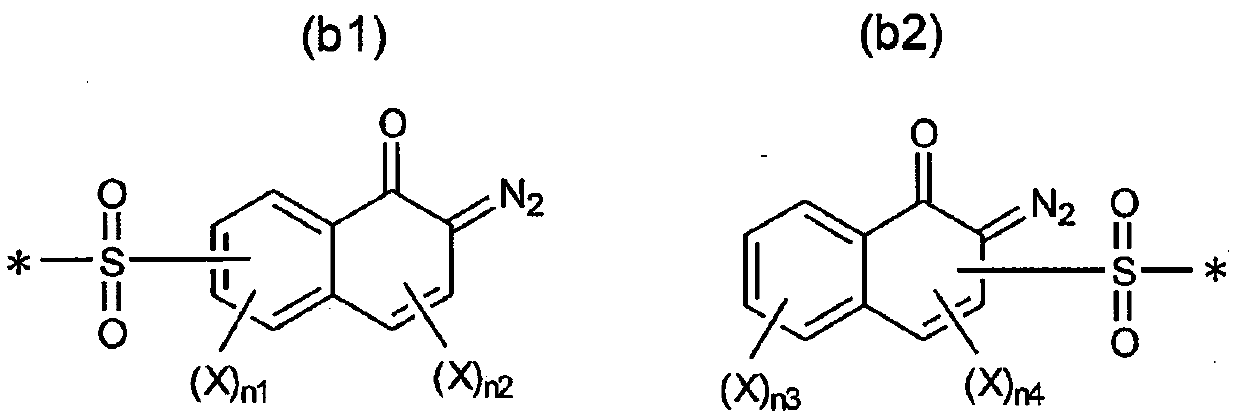
Radiation-sensitive composition and 1, 2-naphthoquinone-2-diazidesulfonic acid derivatives
A radiation-sensitive composition technology, applied in the field of radiation-sensitive composition and 1,2-naphthoquinone-2-diazidesulfonic acid derivatives, can solve the problems of insufficient radiation sensitivity and achieve excellent radiation Effects of Sensitivity and Resolution, Excellent Voltage Hold Characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0138] Hereinafter, the present invention will be described more specifically based on examples. However, the present invention is not limited to the following examples. In the following description, unless otherwise specified, "parts by mass" are described as "parts".
[0139]
[0140] For the polymer (A), Mw and Mn were measured by gel permeation chromatography (GPC) under the following conditions. In addition, the molecular weight distribution (Mw / Mn) was calculated from the obtained Mw and Mn.
[0141] Device "GPC-101" (manufactured by Showa Denko Co., Ltd.)
[0142] GPC column: bonded GPC-KF-801, GPC-KF-802, GPC-KF-803, and GPC-KF-804 (manufactured by Shimadzu GLC)
[0143] Mobile phase: tetrahydrofuran
[0144] Column temperature: 40°C
[0145] Flow rate: 1.0mL / min
[0146] Sample concentration: 1.0% by mass
[0147] Sample injection volume: 100μL
[0148] Detector: Differential refractometer
[0149] Standard material: monodisperse polystyrene
[0150] 〔...
Synthetic example 1
[0152] 7 parts of 2,2'-azobis(2,4-dimethylvaleronitrile), 200 parts of propylene glycol monomethyl ether acetate, 13 parts of methacrylic acid, 40 parts of glycidyl methacrylate, 10 parts of α-methyl-p-hydroxystyrene, 15 parts of N-cyclohexylmaleimide, 12 parts of tetrahydrofurfuryl methacrylate and 10 parts of n-lauryl methacrylate parts, and 3 parts of α-methylstyrene dimer as a molecular weight modifier, slowly start stirring after nitrogen substitution. After raising the reaction solution to 60° C., the temperature was maintained for 5 hours to obtain a polymer solution containing a copolymer (A-1). The copolymer (A-1) was deposited and precipitated using 900 parts of hexane to the obtained polymer solution. The copolymer (A-1) was separated from the liquid in which the copolymer (A-1) was precipitated, and propylene glycol monomethyl ether acetate was added to the copolymer (A-1) to obtain a copolymer containing 30% by mass of the solid content concentration. The polyme...
Synthetic example 2
[0155] The synthesis scheme of Synthesis Example 2 is shown below.
[0156]
[0157]In a flask equipped with a cooling tube and a stirrer, 0.187 g (0.1 mol) of 6-amino-2-naphthoic acid (A1938 of Tokyo Chemical Industry Co., Ltd.) was dissolved in 100 g of propylene glycol monomethyl ether acetate, and 50 g of After water, 233.0 g of chlorosulfonic acid and 335.7 g of 1,1,2,2-tetrachloroethane were mixed, stirred at 20° C. for 1 week, and the water layer was removed for column separation to obtain compound (b-1 ).
[0158] The obtained compound (b-1) was stirred in 100 g of concentrated sulfuric acid at 400°C for 72 hours, the obtained reaction liquid was added dropwise to water, and the precipitate was filtered to obtain the compound (b-1').
[0159] 0.347g (0.1mol) of compound (b-1') was dispersed and dissolved in 150g of water, 10g of concentrated hydrochloric acid was added, 23.5g of 30% sodium nitrite aqueous solution was added dropwise at about 10°C for 1 hour, and tw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com