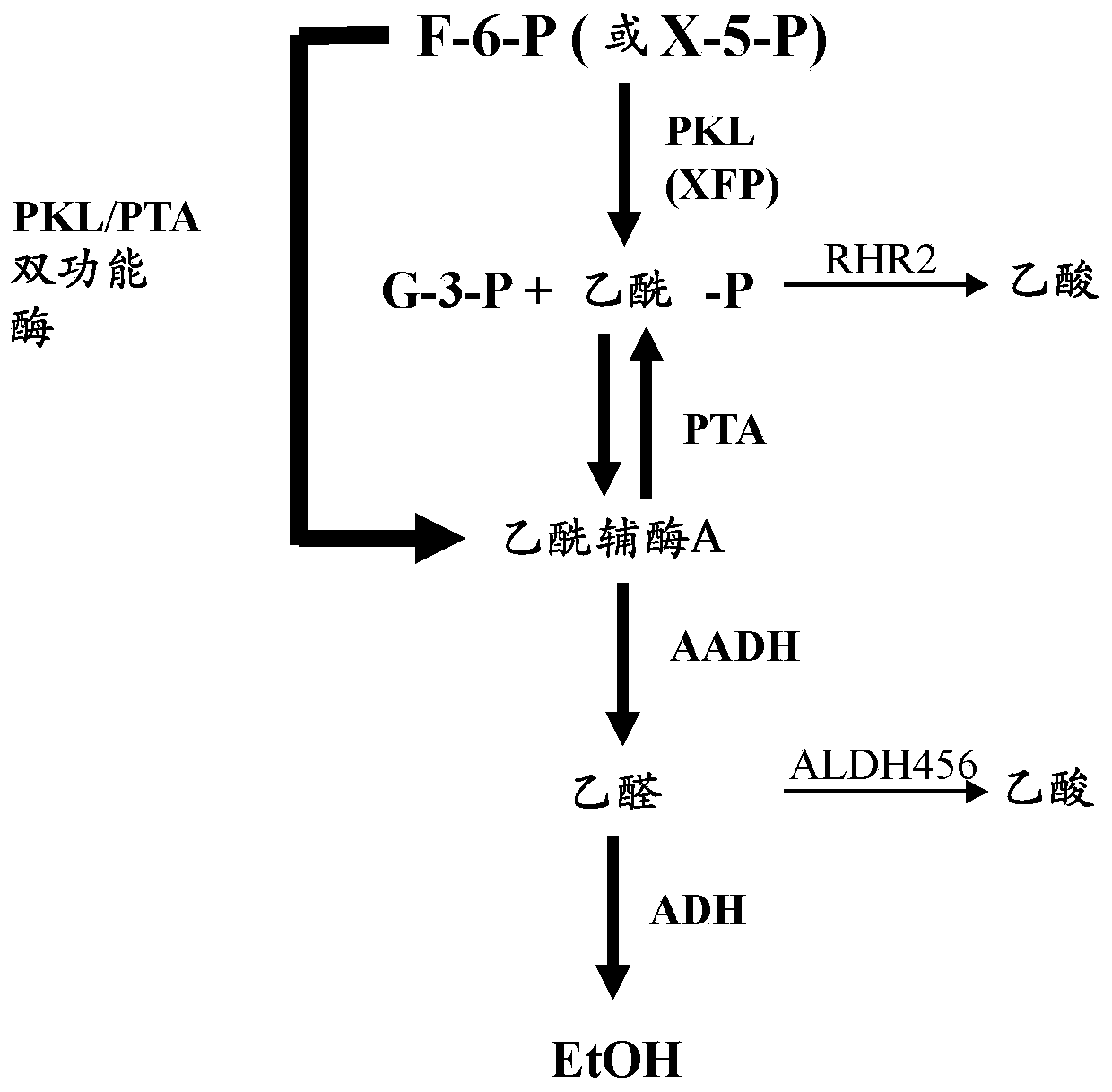
Bifunctional phosphoketolase-phosphotransacetylase fusion polypeptides
A technology of phosphotransacetylase and phosphoketolase, applied in the direction of transferase, acyltransferase, enzyme, etc., can solve the problems affecting the production rate of ethanol, unfavorable and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0165] Materials and methods
[0166] Liquefaction preparation
[0167] The liquefied product (ground corn slurry) was added by adding 600ppm urea, 0.124SAPU / g ds FERMGEN TM 2.5X (acid fungal protease), 0.33GAU / g ds glucoamylase (Trichoderma glucoamylase variant), and 1.46 SSCU / g ds GC626 (Aspergillus alpha-amylase), adjusted to pH 4.8 to prepare.
[0168] Preparation of non-liquefied cornmeal substrate
[0169] Non-liquefied corn flour was prepared by adding desalinated water to corn flour obtained by grinding dry corn kernels and adjusting the pH to 4.8. In addition, add 600ppm urea, 0.124SAPU / g ds FERMGEN TM 2.5X (acid fungal protease), 0.22 mg / g ds TrGA (Trichoderma glucoamylase), and 0.033 mg / g ds AcAA (Aspergillus alpha-amylase).
[0171] Yeast cells were inoculated into 2 mL of YPD in a 24-well plate and cells were grown overnight to reach an OD between 25-30. Aliquots of 2.5 mL of liquefaction were added to serum vials (Chemglass, C...
example 2
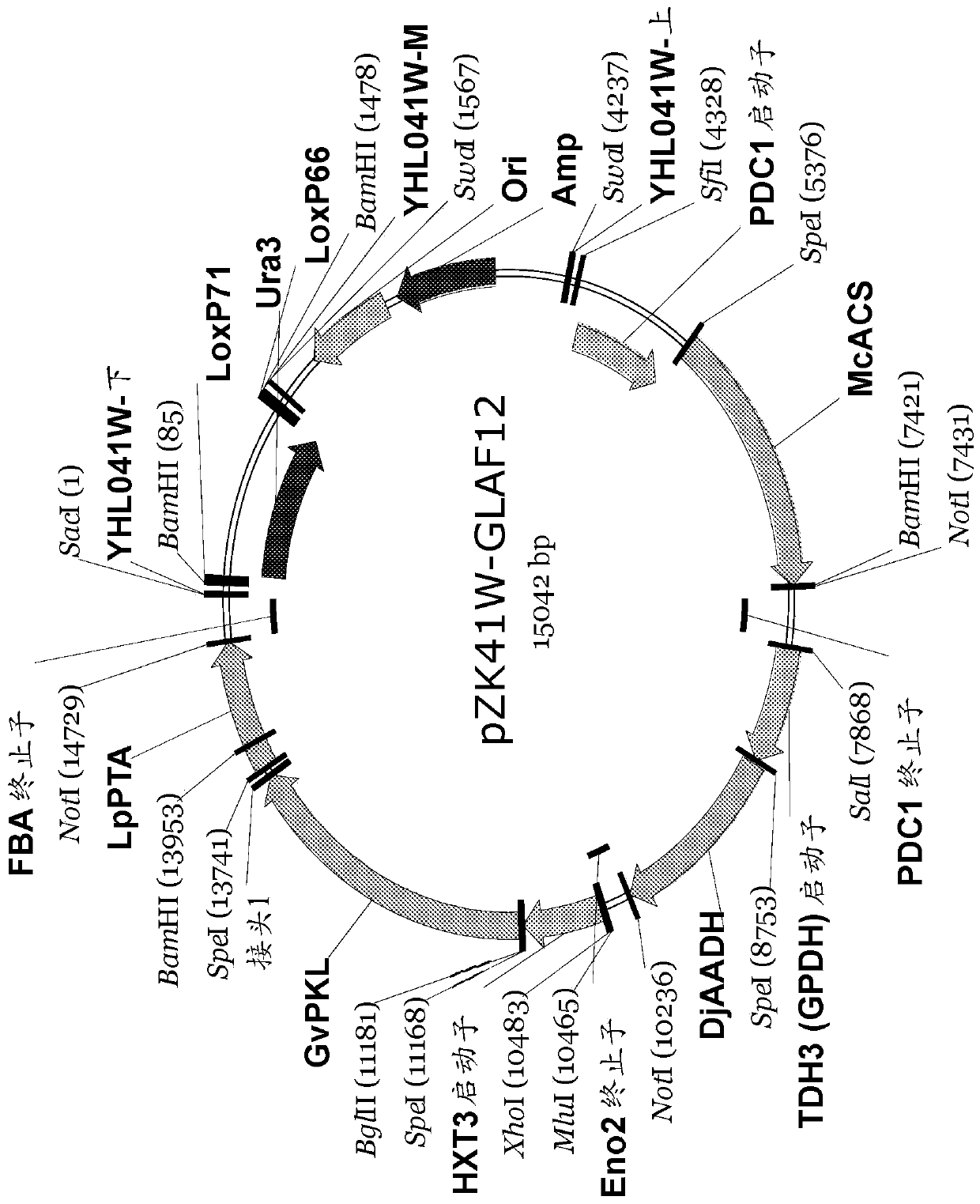
[0185] Plasmid pZK41W-GLAF12 with PKL-PTA fusion gene 1
[0186] The synthetic phosphoketolase and phosphotransacetylase fusion gene 1GvPKL-L1-LpPTA comprises a phosphoketolase (GvPKL; SEQ ID NO: 1) and the codon-optimized coding region of phosphotransacetylase (LpPTA; SEQ ID NO:2) from Lactobacillus plantarum. The amino acid sequence of the resulting fusion polypeptide is figure 2 (SEQ ID NO:5), linker regions are shown in bold italics:
[0187] image 3 Plasmid pZK41W-GLAF12 is shown containing three cassettes to express the GvPKL-L1-LpPTA fusion polypeptide, acetylated acetaldehyde dehydrogenase (DjAADH) from Desulfospira joergensenii and acetyl-CoA synthase (McACS) from Methanosa manecalis . Both DjAADH and McACS were codon optimized. The expression of GvPKL-L1-LpPTA is under the control of HXT3 promoter and FBA1 terminator. The expression of DjAADH is under the control of TDH3 promoter and ENO2 terminator. The expression of McACS is under the control of PDC1 promo...
example 3
[0191] Plasmid pZK41W-GLAF22 with PKL-PTA fusion gene 2
[0192] Synthetic GvPKL-L2-LpPTA comprised the same phosphoketolase and phosphotransacetylase as in Example 2 joined with a synthetic linker 2 (L2; SEQ ID NO 4). The amino acid sequence of the resulting fusion polypeptide is Figure 4 (SEQ ID NO:6), linker regions are shown in bold italics. Plasmid pZK41W-GLAF22 contains a cassette to express GvPKL-L2-LpPTA. Plasmid pZK41W-GLAF22 (not shown) is similar to plasmid pZK41W-GLAF12 but has a different linker.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com