External preparation of simvastatin and application of external preparation
A technology for external preparations and simvastatin, which is applied in the field of external preparations of simvastatin, can solve the problems of pigmentation, repeated treatment, skin loss, etc., and achieves the effects of increasing aggregation concentration, reducing side effects, and avoiding side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
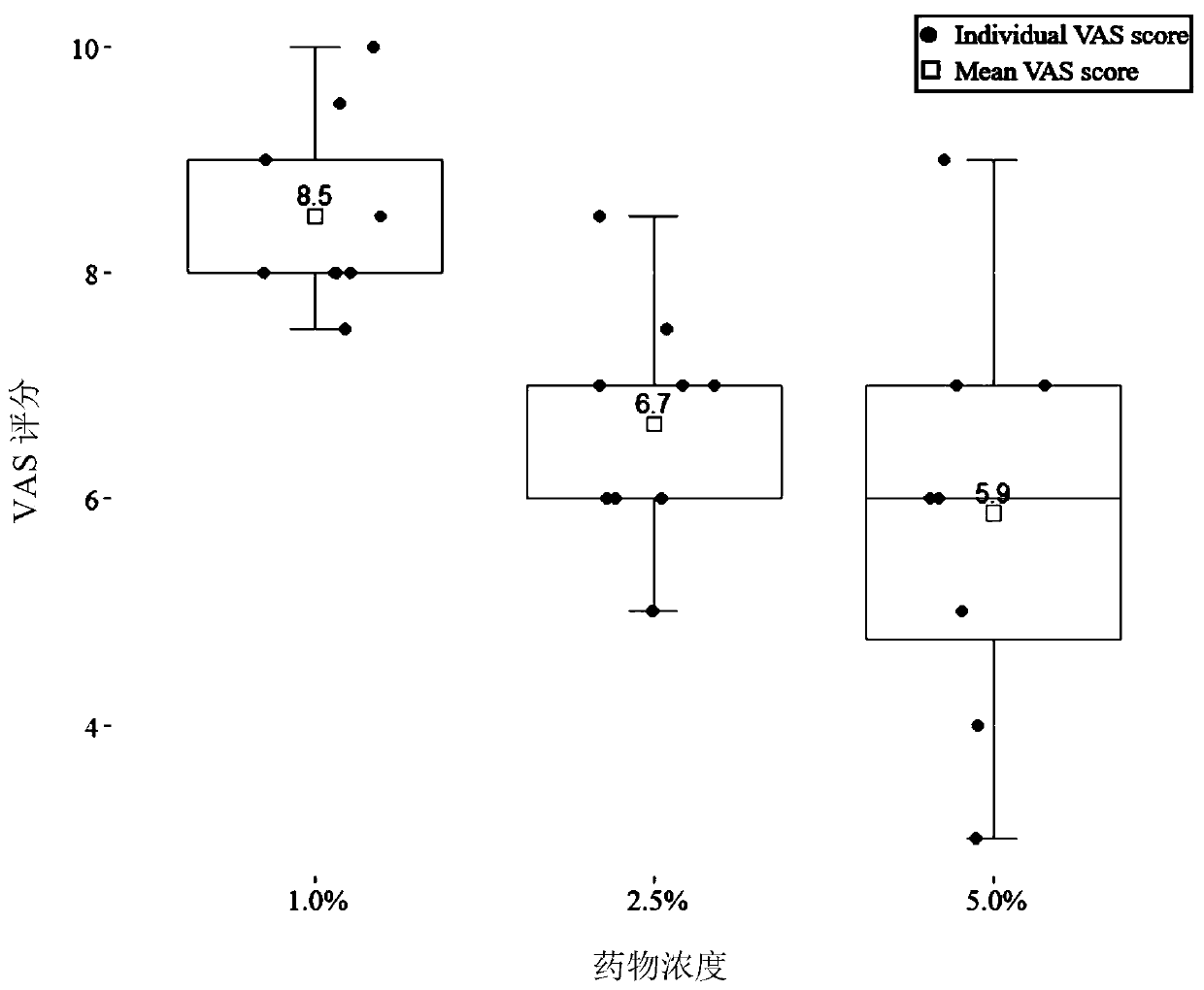
[0054] The present embodiment is 1% simvastatin emulsifiable cream, and formula is as follows:
[0055] Table 1 temporary preparation 1% simvastatin cream (specification: 20g / box)
[0056] product name quantity unit Simvastatin 50 gram stearic acid 573 gram Sodium dodecyl sulfate 10 gram Ethylparaben 5 gram Glyceryl monostearate 333 gram light liquid paraffin 104 ml Triethanolamine 10 ml distilled water 3265 ml glycerin 625 gram Laurocaprazine 25 ml Co-made 5000 gram
[0057] Preparation method: Take glyceryl monostearate, stearic acid, and light liquid paraffin, heat and melt, add ethyl paraben, stir to dissolve, and keep warm at about 80°C. Heat distilled water to boil, add sodium lauryl sulfate, simvastatin, glycerin, grind well, and keep warm at about 80°C. Filter the oil phase into the water phase, stir for 15 to 20 minutes, add triethanolamine and laurocaprazine, an...
Embodiment 2
[0059] The present embodiment is 2.5% simvastatin emulsifiable cream, and formula is as follows:
[0060] Table 2 temporary preparation 2.5% simvastatin cream (specification: 20g / box)
[0061]
[0062]
[0063] Preparation method: Take glyceryl monostearate, stearic acid, and light liquid paraffin, heat and melt, add ethyl paraben, stir to dissolve, and keep warm at about 80°C. Heat distilled water to boil, add sodium lauryl sulfate, simvastatin, glycerin, grind well, and keep warm at about 80°C. Filter the oil phase into the water phase, stir for 15 to 20 minutes, add triethanolamine and laurocaprazine, and continue stirring until it condenses to obtain the product.
Embodiment 3
[0065] The present embodiment is 5% simvastatin emulsifiable cream, and formula is as follows:
[0066] Table 3 temporary preparation 5% simvastatin cream (specification: 20g / box)
[0067] product name quantity unit Simvastatin 250 gram stearic acid 573 gram Sodium dodecyl sulfate 10 gram Ethylparaben 5 gram Glyceryl monostearate 333 gram light liquid paraffin 104 ml Triethanolamine 10 ml distilled water 3065 ml glycerin 625 gram Laurocaprazine 25 ml Co-made 5000 gram
[0068] Preparation method: Take glyceryl monostearate, stearic acid, and light liquid paraffin, heat and melt, add ethyl paraben, stir to dissolve, and keep warm at about 80°C. Heat distilled water to boil, add sodium lauryl sulfate, simvastatin, glycerin, grind well, and keep warm at about 80°C. Filter the oil phase into the water phase, stir for 15 to 20 minutes, add triethanolamine and laurocaprazine, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com